Quantifying the value of LIS, analytics, and data sharing in today’s value-based healthcare environment
Historically healthcare systems have used financial methods to compare spending requests that were in competition for the same dollars. Most of these techniques did not contain a component of quality or improved patient care. Data was not available to associate dollars with these components in order to represent a true picture of the proposal. Most, if not all, spending requests came from individual departments. The result was often that funding was granted, say, to a parking garage project because the cash return was easy to demonstrate, but not to, say, the automation of the chemistry lab, because return on investment was less clear-cut in the eyes of decision makers.
Since 2011 the government and payers have pushed to reduce cost throughout the healthcare delivery system, while at the same time improving the quality of care and patient outcomes. In the current environment, to effectively manage patient outcomes, the provider, patient, and many hospital departments must coordinate the patient care.
Along with the government push for cheaper, better patient care has come the push to establish systems of computerized health records. Information that had primarily been on paper has begun moving to electronic form.
As overall healthcare evolves, the laboratory must also evolve to maintain its value in the healthcare organization. Laboratories can keep a seat at the decision maker’s table by rigorously analyzing laboratory data to detect clinical patterns that can be used to accelerate diagnosis or close care gaps.
The first step in justifying a new laboratory project or system should be evaluating its usefulness and alignment with other organizational priorities. Many different tools exist that can be used for this purpose. Two of these tools, the Purpose-based Alignment Model and the Business Model Canvas, can be used to identify projects, processes, and systems that support the organization’s business objectives and contribute to the laboratory value within the organization.
Innovative tools to identify projects and systems
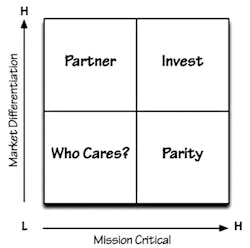
The Purpose Alignment Model,1 or PAM, (Figure 1), created by business/technology expert Niel Nickolaisen, is a method for aligning business decisions, processes, and feature designs around purpose. The purpose of some decisions and designs is to differentiate the organization in the market; the purpose of most other decisions is to achieve and maintain parity with the market. Those activities that do not require operational excellence either necessitate finding a partner to achieve differentiation or do not deserve much attention.
The PAM ranks systems or projects by market differentiation and criticality for the organization (termed “mission critical”). Items falling in the top right-hand box are items that should be prioritized. Historically, lab projects and purchases would fall in the parity box, which is high on the mission critical scale, but lower on the differentiation scale.
When should the PAM be used? Purpose alignment works well when you need to do these things:
- define business and IT strategic and tactical plans
- align IT with business priorities
- evaluate, plan, and implement large system projects
- filter and design features and functionality
- manage project scope
- reduce resistance to process improvements
- reduce waste by improving focus and resource allocation.
The PAM provides a simple way to determine what activities to concentrate on, and how to deliver them. Ranking items by criticality and market differentiation removes factors that merely act as distractions to decision making and helps the team focus.
Differentiation items create a competitive advantage and link directly to the core business. In healthcare, the business value (differentiation) is often derived from improving care outcomes and reducing costs. This requires the lab to consider costs outside its responsibility and consider how lab results can reduce the cost of care or improve the quality of care. (The best differentiators will do both.) What can the laboratory do to meet goals such as improving quality of care? Reducing length of stay? Closing gaps in chronic disease management?
The Business Model Canvas, or BMC2 (Figure 2) is a strategic management and Lean startup template for developing new or documenting existing business models. It is a visual chart with elements describing a firm’s or product’s value proposition, infrastructure, customers, and finances.
The BMC is designed to work with cross-functional teams to understand their organization’s existing business model and test out potential new business models. Once leaders of the organization have the current business model mapped out, they develop assumptions for the future. These are business assumptions on which to build. For example, here are assumptions for the laboratory:
- Decreasing reimbursement schedules will have a significant negative impact on lab test profitability.
- Test volume from chronic diseases will rise due to an aging population.
Here are assumptions for the health system:
- MACRA will incentivize healthcare providers to provide greater value to government-insured patients, driving the demand for analytics tools.
- The percent of doctors employed by the health system will remain stable or rise.
Examples of front-line innovation
The first two examples of innovation given below started as laboratory initiatives. The third was a CEO-initiated strategic direction to encourage innovation throughout the entire organization.
Example one: Promoting improved utilization of laboratory testing. This case study4 describes five years of experience with interventions to improve laboratory test utilization at an academic medical center (University of Iowa). Results included interventions to tackle overutilization of automated tests, which included limits on repetitive ordering by placing messages in the EHR system regarding when the test was last performed and a link to the last results. These interventions proved to be effective in reducing repetitive ordering. The high-frequency laboratory tests showing the biggest declines in order volume post intervention were serum albumin (36 percent) and erythrocyte sedimentation rate (17 percent). Targeted alerts reduced duplicate orders of germline genetic testing and orders of hepatitis B surface antigen within two weeks of hepatitis B vaccination.
Another category of laboratory testing that was targeted to improve laboratory utilization was low-volume but high-cost tests such as panels for genetic testing or autoantibodies. In some cases, these panels may have direct costs of thousands of dollars (sometimes paid directly to external reference laboratories by hospitals, clinics, or laboratories) yet have poor reimbursement by payers. Lab leaders developed “laboratory test formularies” that place tiered restrictions on ordering of certain tests. Restrictions for specific testing included need for pre-approval by pathology or another designated group prior to ordering or limitation of ordering of certain tests to specific specialties (e.g., esoteric coagulation tests by hematology/oncology specialists). Introduction of restrictions for 170 high-cost send-out tests resulted in a 23 percent decline in order volume.
Two quality improvement projects within the Pathology department focused on misorders of laboratory tests with similar-looking names. Targeted interventions reduced misorders involving several “look-alike” tests: 1,25-dihydroxyvitamin D, 25-hydroxyvitamin D; manganese, magnesium; beta-2-glycoprotein, beta-2-microglobulin.
Example two: Early detection of acute kidney injury (AKI).5,6 AKI is an under-recognized and under-diagnosed disease. Diagnosis delay can result in a six- to 30-fold increase in in-hospital mortality, and hospitalization costs increased by $4,000 to $10,000/day/patient.
This was the issue that Northwell Health Laboratories set out to address by monitoring inpatient creatinine results. Delta creatinine detects 99.8 percent of all AKI patients. That means a 50 percent rise in creatinine, according to the relative criteria, or an absolute 0.3 mg/dl increase from the prior baseline minimum value. This has better sensitivity and specificity than other clinical criteria and can be applied in routine hospital practice.
The project involved issuing an alert within the health system’s electronic records to doctors when creatinine levels surpass the benchmarks that could be indicators of AKI. When the alert was first implemented in 2014, the actual identification of AKI within Northwell was around five percent of hospital inpatients. By 2016, after the early warning system alert was implemented, the number of patients identified with AKI had risen to 13 percent.
This early-warning system for AKI helped physicians intervene earlier, improving outcomes and reducing length of stay. Using literature estimates of a two-day drop in length of stay per case for patients treated quickly for AKI, the imputed annual savings at the pilot site, Forest Hills Hospital, alone were $875,000 based on 2,190 avoided hospital days.
In addition to better outcomes and reduced length of stay, the secondary inpatient diagnosis of acute kidney injury can be added to the bills the hospital sends to payers, and adds an average of $700 in hospital revenue per patient. The new lab-driven system increased monthly secondary diagnoses of AKI from an average of 615 in 2014 to 930 in 2015. That adds up to an estimated $220,500 monthly jump in revenue for Northwell Health, a nearly $2.65 million annual increase.
Several data challenges were encountered during this project, which highlights the importance of interoperability throughout the system:
- lack of access to administrative data which can be readily linked to laboratory data
- difficulty in calculating total cost of care and therefore the effect of laboratory intervention
- laboratory data not being linked to other data sets such as pharmacy and claims data
- lack of eMPI preventing linking of inpatient laboratory data to outpatient laboratory data and longitudinal follow-up of patients.
Example three: Overcoming bureaucracy and breaking down silos via strategic innovation.7 These objectives are perhaps especially critical in healthcare. To address these challenges, leaders at the Hospital for Sick Children (Sick Kids) in Toronto, Ontario, elevated innovation to a strategic direction and engaged a business consulting company to help devise a full system needed to spur innovation. The resulting system has three major components:
- An innovation blueprint that detailed the types of innovations the organization wants to encourage. SickKids prioritized encouraging doctors, nurses, and clinicians to look for unmet needs they could address, rather than wait for solutions from IT or top management. That required creating a focus group with 25 front-line healthcare workers to discover and catalog key “jobs to be done” (such as reducing the length of hospital visits), surveying all 5,000 employees, and training most of them on how to integrate the innovation system into their daily practices.
- An innovation pipeline that brought ideas from concept to reality. This involved establishing a new 18-member Central Innovation Group of leaders from different areas of the hospital, a team that was tasked with prioritizing and advancing ideas and projects through various stages. The team helped innovators test prototypes, make adjustments, and then scale to a wider population.
- An innovation culture that features the right people, in the right roles, speaking a common language of innovation. A key enabler of this culture was the establishment of a $250,000 Innovation Fund to provide seed money for promising ideas. Now, instead of being stalled by permission hurdles that suppress initiative, promising new ideas can be funded, fast-tracked, and prototyped.
Following are two of many innovations that grew out of this strategy:
- A pain reporting application, Pain Squad, encourages pediatric cancer patients to record pain levels. Whereas pain reporting with paper diaries yielded compliance rates below 50 percent and Web-based diaries yielded 70 percent, Pain Squad has boosted rates to more than 90 percent.
- A simple one-screen billing app for the iPad has improved billing procedures. Canada has a single-payer system, but hospitals still must track all procedures so that the hospital and its doctors are properly paid. At SickKids, only 30 percent of procedures were being filed accurately and on time. With a $10,000 grant from the Innovation Fund, the app went from idea to pilot in three months. It is now used throughout several departments in the hospital. In urology, for example, billing compliance rates went from below 50 percent to higher than 99 percent.
Evaluating functionality
After identifying potential projects using the PAM and BMC tools, lab leaders will have a better understanding of the functionality required for the project.
It is a given that Lab IT systems should provide management data that can boost lab productivity, workflow, and accuracy. In more recent years, additional expectations such as analytics for turnaround time, physician utilization, staffing workload, auto-validation percentages, and other quality measures have been added to the list of expectations.
The Association for Pathology Informatics has created an LIS Functionality Assessment Toolkit that contains a comprehensive list of available LIS features. Appendix III of the toolkit is a list of the elements that should be included in the total cost of ownership. The toolkit is available to the public and can be accessed at https://www.pathologyinformatics.org/lis_toolkit.php.
In addition to traditional IT functionality, data analytics and interoperability will be required for the laboratory to make a greater positive impact on patient care and cost savings. To effectively measure total quality of care and the total cost to provide that care, data silos must be combined into one master data base or, alternatively, communicate with one another to exchange data (interoperability) that can track all aspects of the patients care and the associated cost.
Molecular and genetic advances are emerging that require advanced algorithms, interpretations, and big data technology that older technology cannot handle. Because these tests produce large quantities of data, new technologies will require a “Big Data” component that allows for mining and evaluation of large quantities of unstructured data. Even if an institution doesn’t perform these assays now, that doesn’t rule out that it might perform them in the future.
Financial measurements
Once lab leaders have developed the concept and evaluated the functionality required, they can use traditional financial tools to prioritize the projects. The most common financial methods used for analyzing the purchase of equipment and services are: 1) payback (or break even) analysis; 2) return on investment (ROI); and 3) net present value (NPV).
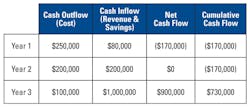
Payback analysis refers to the period of time required to recoup the funds expended in an investment, or to reach the break-even point. Payback period is usually expressed in years. In the example given in Table 1, the payback year would be Year 3.
Net cash flow doesn’t have to be just revenue. It could be savings, for example:
- labor savings if implementing a system that performs auto-verification.
- cost-of-testing savings if the system can monitor and reduce inappropriate test utilization.
- cost-of-patient-care savings if the system can perform algorithms that detect care gaps that, when identified, lead to more efficient and effective patient care.
ROI is a simple calculation that is used by organizations to compare capital expenses or projects that are competing for the same investment dollars. The simplest ROI calculation would be the manufacture of a laboratory instrument. If the instrument sold for $100,000 and cost $90,000 to manufacture, the ROI would be 11 percent: return on investment = (revenue − cost of goods sold) / cost of goods sold; ($100,000 – $90,000) / $90,000 = .11 or 11 percent.
Unfortunately, ROI in healthcare organizations are not as straightforward as revenue and cost. Gain from the investment is normally substituted for revenue, or it could be a combination of both. Gain from the investment could be revenue, labor savings, reduced length of stay, or fewer Emergency Department visits. Whatever the gain, it must be converted to a dollar value: return on investment = (gain from investment – cost of investment) / cost of investment.
The Total Cost of Ownership (TCO), not just purchase price, should always be used for the above calculations. TCO will include the licensing fees for the system, the installation fees, training fees, yearly maintenance fees, and the cost of hardware and software upgrades. Lab leaders should not forget the costs to migrate data from existing systems, or ongoing maintenance of existing systems for data access. Some institutions also include the labor costs of their employees for training and implementations. These resources should be understood, even if not included in the TCO calculation.
NPV is a financial assessment tool that considers that the value of a dollar today and the value of a dollar in the future are not equal. If a project has income over a long period of time, for example 30 years, the future dollars are discounted at a notional rate determined by the project stakeholders. This calculation is used for capital projects such as buildings that have paybacks of many years. It can also be used to compare contractor bids with long payment schedules.
Internal Rate of Return (IRR) is the rate at which the project breaks even. It’s commonly used by financial analysts in conjunction with NPV. That’s because the two methods are similar but use different variables. NPV assumes a particular discount rate for a company, then calculates the present value of the investment. IRR calculates the actual return provided by the project’s cash flows, then compares that rate of return with the company’s hurdle rate (how much it mandates that investments return). If the IRR is higher, it’s a worthwhile investment.
Get started
We have discussed innovative tools that can be used to identify projects and systems that are in alignment with a healthcare organization’s business model and goals. The two laboratory examples of innovation proved that being able to recognize patterns in clinical data can be used to make more informed decisions which resulted in:
- reduced lab volume from proper test utilization; and
- improved patient outcomes and a reduced length of stay.
Functionality checklists for information technology projects are included as well as formulas for the financial analysis of projects and systems.
Don’t wait to put these techniques to work in your organization! Remember to:
- link laboratory strategy with the total health system strategies
- work with departments throughout the health system to identify
- opportunities share and analyze data to identify opportunities and build lab value.
REFERENCES
- McDonald KJ. Purpose based alignment model (White Paper) https://www.alaska.edu/files/pathways/WhitePaperonPurposeBasedAlignmentModel.pdf.
- Barquet PAB, Cunha VP, Oliveira MG, Rozenfeld H. Business model elements for product service system. In: Hesselbach J., Herrmann C. (eds) Functional Thinking for Value Creation. 2011. Springer, Berlin, Heidelberg.
- Murphy M. A healthcare Innovator’s roadmap: 4 steps for developing a business model. Vector. Boston Children’s Hospital’s Science and Innovation Blog https://vector.childrenshospital.org/2017/01/innovators-roadmap-developing-business-model/.
- Krasowski MD, Chudzik D, Doelzal L, et al. Promoting improved utilization of laboratory testing through changes in an electronic medical record: experience at an academic medical center. BMC Med Inform Decis Mak. 2015;Feb 22;15:11.
- Kothari T. Standardizing early detection of acute kidney injury in an integrated delivery health system. https://www.executivewarcollege.com/wp-content/uploads/Kothari.wed_.9am.FINAL_.pdf.
- Crawford JM. The new game in healthcare: keeping patients healthy and out-of-hospital; precision medicine; the role of lab testing. http://www.labqualityconfab.com/wp-content/uploads/tue.CRAWFORD.5pm.FINAL2_.pdf.
- Duncan DS. Driving front line innovation in health care. Harvard Business Review. https://hbr.org/2013/04/driving-front-line-innovation.
Linda Newman, MT(ASCP), serves as Director of Product Marketing for ELLKAY. Linda’s prior experience includes senior management positions in both start-ups and Fortune 100 corporations specializing in market assessment, product development and launch, and support of technology products and services in the healthcare marketplace. She has also managed the chemistry laboratory of a 1,200-bed hospital. She holds a BS degree in Medical Technology and is ASCP-certified.