Confronting the challenges of influenza-like illness
CONTINUING EDUCATION
To earn CEUs, visit www.mlo-online.com under the CE Tests tab.LEARNING OBJECTIVES
1. Describe the healthcare cost burden of ILI in the United States and identify the symptoms and at-risk populations.
2. Identify the advances in multiplex molecular testing for ILI that have improved healthcare outcomes.
3. Describe past studies and their outcomes as related to improved rapid multiplex testing.
4. Discuss future opportunities for research in rapid multiplex testing.
Influenza-like illness (ILI) is a substantial clinical and economic burden on patients, healthcare providers, and the broader healthcare system. Depending on the pathogenicity of the viral strain and the effectiveness of the vaccine, there are typically between nine million and 36 million influenza cases annually in the United States, resulting in 140,000 to 710,000 hospitalizations.1 However, influenza represents a small percentage of the hundreds of millions of upper respiratory infections (URIs) that occur annually in the U.S. alone.2,3 This broader group of infections accounts for more healthcare provider visits than any other acute condition annually and results in almost 50 million lost days from work and school.2,4,5 While the literature lacks reliable, contemporary data on the economic costs of URIs and more specifically ILI, it is estimated that direct and indirect costs combined likely exceed $100 billion each year.3,6
In addition to its high social and economic costs, ILI can lead to severe health consequences for individual patients, particularly among at-risk populations including the very young, the elderly, and the immunocompromised. Influenza alone is responsible for 12,000 to 56,000 deaths annually in the U.S.1 And beyond the measurable mortality and morbidity of ILI, accurate, rapid diagnosis of the patient’s condition also has substantial implications for key institutional quality metrics such as antimicrobial stewardship and infection control. This article will review the diagnostic challenges associated with ILI, the implications of missed or delayed diagnosis, and new diagnostic tools that may help address these challenges. Finally, areas for future research with respect to ILI diagnosis and patient management will be discussed.
Clinical presentation and differential diagnosis
ILI is a condition that presents with fever, cough, sore throat, shivering, chills, malaise, body aches, and/or nausea and is often associated with rapid onset. Frequent causes include the common cold and influenza, but ILI can be caused by more than 20 different viral and bacterial pathogens with overlapping and non-specific presentations. This complicates accurate, timely diagnosis.
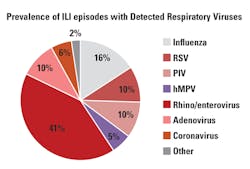
More than 200 subtypes of viruses cause the common cold. While rhinoviruses represent a plurality of causative pathogens (30 percent to 50 percent of colds), other infectious agents are also implicated: coronaviruses (10 percent to 15 percent); influenza viruses (five percent to 15 percent); respiratory syncytial viruses (RSV, ~10 percent); parainfluenza viruses (PIV, ~ five percent); enteroviruses (< five percent); and human metapneumovirus (hMPV).7 Additionally, the cause of 20 percent to 30 percent of common colds is unknown. Given the similar presentation associated with these viruses, it is not possible to establish the causative pathogen based on clinical diagnosis alone.
For instance, the differential for RSV in adults includes influenza and PIV. In infants it is even broader, including influenza, PIV, hMPV, rhinovirus, coronavirus, human bocavirus, and adenovirus. Studies have shown that RSV infection develops annually in three percent to seven percent of healthy older adults, may contribute to excess wintertime mortality previously attributed to influenza, and is a leading cause of hospitalization in young patients.8-10
Even the diagnosis of influenza can be confounded by the overlapping syndromes of ILI. A meta-analysis that reviewed the precision and accuracy of symptoms and signs of flu in adult patients over 60 years of age concluded that “clinical findings identify patients with influenza-like illness but are not particularly useful for confirming or excluding the diagnosis of influenza.”11
Rapid and accurate diagnosis of the causative pathogen(s) for ILI is critical for informing patient management and selecting proper treatment, particularly in high-risk and hospitalized patients. Beyond direct patient impact, appropriate management of ILI can also help address key quality metrics such as infection control and antimicrobial stewardship.
High-risk patient populations
ILI poses a significant risk in immunosuppressed and immunocompromised patients, including hematopoietic stem cell transplant patients, solid organ transplant recipients, and patients receiving chemotherapy. Influenza, RSV, PIV, hMPV, adenovirus, and rhinovirus are associated with increased morbidity and mortality in these patient populations.12-14 Rapid, accurate diagnosis is an important component of patient management in these populations as it helps direct appropriate antiviral and/or antibiotic therapy and can inform decisions about timing of transplant or additional therapy.15 Current practice guidelines support testing for a wide range of suspected respiratory pathogens in these high-risk populations.16-18
Patients in the intensive care unit (ICU) setting are also particularly vulnerable to complications from ILI. Viral pathogens including influenza, RSV, PIV, hMPV, coronavirus, and rhinovirus and are all associated with severe pneumonia, requiring management in the ICU.19 And while guidelines for respiratory virus testing in the ICU population are undefined, a recent study showed that fewer than half of ICU patients with hospital- or community-acquired pneumonia were tested for viral pathogens. Among the patients that were tested, overall prevalence of viral infection was 28 percent, with 63 percent of the identified pathogens being other than influenza or RSV.20
The pediatric population is also at higher risk of adverse outcomes from ILI, as respiratory tract infections account for increased mortality and morbidity in patients who are less than five years of age.21 RSV and PIV are the two leading causes of hospitalization for respiratory tract illness in young children, and RSV is estimated to cause more deaths in patients less than one year of age than any infectious agent other than malaria.9,22
Infection control
As healthcare payment models in the United States continue to shift away from fee-for-service and toward more capitated structures, managing overall cost-of-care is becoming increasingly important for providers who carry financial risk associated with avoidable readmissions and treatment of healthcare-acquired infections. These shifting financial incentives are leading to increased emphasis on and investment in infection control practices within the hospital. The U.S. Centers for Disease Control and Prevention (CDC) guidelines related to ILI recommend infection control practices that include patient isolation, targeted triaging, cohorting, and barrier protections.23,24
For infection control with suspected or confirmed influenza patients, the CDC recommends adherence to standard contact and droplet precautions as well as isolation and/or cohorting.24 RSV is highly contagious and associated with serious healthcare-acquired infections. Infection control measures, including patient isolation or cohorting, limitations on patient transport, and contact and/or droplet precautions, are recommended to limit nosocomial spread, particularly in an outbreak scenario.25,26 Similar precautions are recommended for hospitalized patients with PIV infection, particularly if exposure to immunocompromised patients is possible.13,23 Accurate, rapid diagnosis of the causative agent of ILI is required to appropriately inform these various infection control practices and to manage limited isolation bed space, particularly during peak respiratory virus season.
Antimicrobial stewardship
The CDC reports that annually more than two million illnesses and 23,000 deaths are caused by antimicrobial-resistant (AMR) bacteria in the United States.27 Pervasive inappropriate use of antibiotic therapy is a major contributor to the growing public health crisis of AMR. A recent large, population-based study assessed antibiotic prescribing patterns for more than 185,000 elderly patients who presented in the outpatient setting with a confirmed nonbacterial acute upper respiratory infection. The study showed that 46 percent of patients received an antibiotic prescription, with 70 percent of those receiving broad-spectrum therapy, despite a confirmed nonbacterial infection.28 The literature demonstrates similar results related to misuse and overuse of antibiotics in varying care settings and across diverse patient populations, sometimes resulting in adverse patient outcomes and progressive antimicrobial resistance.29-33
The CDC’s 2013 report on Antibiotic Resistance Threats in the United States led to the creation of a National Strategy for Combating Antibiotic Resistant Bacteria (National Strategy) which noted that one-third to one-half of all antibiotics used in inpatient and outpatient settings are either unnecessary or incorrectly prescribed.34 Inappropriate use of antimicrobial therapy not only contributes to growing AMR, but also places an unnecessary economic burden on the healthcare system, with more than $1.1 billion in annual domestic spending on unnecessary antibiotic prescriptions for respiratory infections in adults.35
One objective of the CDC’s National Strategy is to “develop new diagnostics, including tests that rapidly distinguish between viral and bacterial pathogens and…that can be implemented in a wide range of settings.”34 The CDC report notes: Presently, most diagnostic tests take 24 to 72 hours from specimen collection to results….Thus, treatment decisions are typically required and made before laboratory results are available. As a consequence, patients may be initially treated with antibiotics when none are needed, prescribed an inappropriate antibiotic, or treated with multiple antibiotics when a single antibiotic would have been effective….However, the technological landscape is changing at a rapid pace. The current trend is moving towards clinical presentation or point-of-need diagnostic tests suitable for use during a healthcare visit because they require only minutes.34
New diagnostic tools
Consistent with the CDC’s National Strategy objective of developing new, flexible diagnostic capabilities, multiplex molecular testing is one tool that is now available to help resolve the overlapping clinical presentation of ILI and to address the need for rapid, accurate diagnosis of the causative pathogen. Previously, this type of multiplex molecular testing required advanced technical skills and equipment and was primarily restricted to the high-complexity laboratory setting. However, recent advances by multiple vendors have resulted in the commercialization of FDA-cleared, sample-to-answer platforms that significantly reduce the laboratory and staffing requirements needed to generate highly sensitive molecular results for the wide range of pathogens that are implicated in ILI. These diagnostic platforms have achieved both CLIA moderate complexity and waived status, making them accessible in a range of different care settings.
The past several years have shown rapid growth in the publication of studies reporting on the impact of sample-to-answer, multiplex molecular diagnostics for ILI. These studies have demonstrated the impact this technology can have across multiple care settings and on multiple clinical, quality, and economic outcome measures. Almost all of the studies have shown that multiplex molecular testing provides a more definitive diagnosis through a higher positivity rate while also delivering this result in a significantly shorter turnaround time, providing data in a clinically actionable timeframe.36-39
Rogers et al reported that the implementation of a rapid, multiplex molecular assay in a major children’s hospital led to a significantly higher positive test result rate (77.9 percent vs. 59.8 percent) while also providing a 65 percent reduction in time-to-result compared to a batch, PCR assay.36
Martinez et al reported on their experience with a rapid, multiplex molecular assay for ICU patients compared to conventional batch testing. They reported an average 30.4 hour reduction in mean time from sample collection to reported result. This shorter time-to-result contributed substantial clinical and economic outcome improvements with a reported 10 percent increase in the relative survival rate among the rapid, multiplex testing group. These patients also experienced a three-day reduction in ICU stay, contributing to a more than $8,000-per-patient reduction in the total cost-of-care.38
In perhaps the most rigorously designed study completed to date on rapid, multiplex molecular testing for respiratory pathogens, Brendish et al recently reported the results of a prospective, randomized controlled trial on the use of this technology at the point of care in the emergency department (ED). Consistent with prior reports, this study showed that rapid, accurate results impacted patient management, reduced cost-of-care, and contributed to appropriate infection control precautions. For patients with a positive test result, clinicians were able to stop antibiotics earlier, rather than completing a standard five-to-seven day course. With respect to antiviral therapy, 91 percent of influenza-positive patients in the rapid, multiplex testing group received appropriate, guideline driven antiviral therapy, compared to only 65 percent in the control group. For patients who were admitted to the hospital from the ED, the rapid, multiplex testing group experienced a 1.1-day shorter overall length-of-stay (LoS), contributing to an estimated $500 net cost savings per patient. And twice as many patients in the rapid, multiplex testing group with confirmed respiratory viral infections were isolated compared to the control group.40
These results in the ED have been confirmed in other studies that have shown higher rates of results reported to the patient while still in the ED (51.6 percent vs. 13.4 percent),36 lower hospital admission rates,37 reduced time in the ED by up to 23 percent,38 reduced time to appropriate therapy39 and reduced overall LoS for patients subsequently admitted to the hospital.38
In addition to these direct clinical and patient benefits, many of the studies also show improvements in key quality metrics. Multiple studies have shown reductions in the inappropriate use of antibiotics across a wide range of care settings and patient populations, consistent with CDC guidelines and the public health goal of reducing AMR.36-38 These studies have shown that during peak respiratory virus season, when isolation facilities are at a premium, rapid, multiplex respiratory testing can be used successfully to inform cohorting strategies.39,40 This use of multiplex testing in support of infection control measures is consistent with clinical guidelines and best practices that recommend the “application of rapid diagnostic tests to support clinical decisions involving patient treatment, room selection, and implementation of control measures.”23
Opportunities for future study
The development of multiplex molecular diagnostic tools for ILI continues to accelerate at a rapid pace. And while the literature supporting the adoption of this technology also continues to grow, several gaps remain to be addressed. For example, there is strong evidence to support broad use of this technology in certain patient populations, such as pediatrics, the immunocompromised, and those in intensive care, but the clinical utility of rapid multiplex testing is other patient populations that are vulnerable to complications from ILI (e.g., the elderly), is not as well established. Studies focused on establishing the impact of multiplex testing in these patient populations should be areas of future investigation. Additionally, larger prospective studies appropriately powered to assess the clinical and health economic impact of these technologies would also be beneficial. The current literature suggests that providing rapid, accurate diagnostic results for ILI translates into improved outcomes, better quality metrics, and lower overall cost-of-care, but more robust studies to validate these results would benefit the laboratory community.
For now, what we know for sure is this: ILI is a high-prevalence condition that afflicts all patient populations and results in significant clinical and economic costs. The diagnosis of ILI is challenging, given the overlapping clinical presentation and the broad differential diagnosis that includes both viral and bacterial pathogens. Implementation of guidelines-driven infection control and antimicrobial stewardship interventions are predicated on rapid, accurate diagnosis of the causative agent. This definitive diagnosis is particularly important in high-risk populations such as patients with a suppressed immune system, patients in intensive care, and infants.
Sample-to-answer, multiplex molecular testing is a technology that can help address the challenges associated with the management of ILI. This testing has been shown to improve patient outcomes, reduce total cost-of-care, and support key quality measures such as appropriate antibiotic use and infection control. While there remain opportunities to further strengthen the evidence supporting adoption of this technology, sample-to-answer, multiplex molecular platforms are increasingly viewed as an essential tool in the diagnostic laboratory for the management of ILI. As the American Society for Microbiology (ASM) concluded in its recent white paper on the clinical utility of multiplex tests for respiratory pathogens: “There is no question that multiplex molecular panels provide superior diagnostic performance when compared to conventional methods, and there is a small, but growing, body of evidence that supports their positive impact on patient care and reduction in overall healthcare costs.”41
REFERENCES
- Centers for Disease Control and Prevention. Disease burden of influenza. May 2017. https://www.cdc.gov/flu/about/disease/burden.htm.
- Centers for Disease Control and Prevention. Common colds: protect yourself and others. February 2017. https://www.cdc.gov/features/rhinoviruses/index.html.
- Fendrick AM, Monto AS, Nightengale B, Sarnes M. The economic burden of non-influenza-related viral respiratory tract infection in the United States. Arch Intern Med. 2003;163(4):487-494.
- Turner RB. Epidemiology, pathogenesis, and treatment of the common cold. Ann Allergy Asthma Immunol. 1997;78(6):531-539.
- Centers for Disease Control and Prevention. National Center for Health Statistics. Ambulatory Health Care Data. NAMCS Summary Data, Table 16. April 2017. https://www.cdc.gov/nchs/data/ahcd/namcs_summary/2014_namcs_web_tables.pdf.
- Molinari NA, Ortega-Sanchez IR, Messonnier ML, et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007;25(27):5086-5096.
- Heikkinen T, Järvinen A. The common cold. Lancet. 2003;361(9351):51-59.
- Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289(2):179-86.
- Hall CB. Respiratory syncytial virus and parainfluenza virus. N Engl J Med. 2001;344(25):1917-1928.
- Falsey AR, Hennessey PA, Formica MA, et al. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352(17):1749-1759.
- Call SA, Vollenweider MA, Hornung CA, et al. Does this patient have influenza? JAMA. 2005;293(8):987-997.
- Ison MG. Respiratory viral infections in transplant recipients. Antivir Ther. 2007;12(4 Pt B):627-638.
- Boeckh MJ. The challenge of respiratory virus infections in hematopoietic cell transplant recipients. Brit J Haematol.2008;143(4):455-467.
- Chemaly RF, Shah DP, Boeckh MJ. Management of respiratory viral infections in hematopoietic cell transplant recipients and patients with hematologic malignancies. Clin Infect Dis. 201;59(5):344-351.
- Shah DP, Ghantoji SS, Mulanovich VE, et al. Management of respiratory viral infections in hematopoietic cell transplant recipients. Amer J Blood Res. 2012;2(4):203-218.
- Dignan FL, Clark A, Aitken C, et al. BCSH/BSBMT/UK clinical virology network guideline: diagnosis and management of common respiratory viral infections in patients undergoing treatment for haematological malignancies or stem cell transplantation. Br J Haematol. 2016;173(3):380-93.
- von Lilienfeld-Toal M, Berger A, Christopeit M, et al. Community acquired respiratory virus infections in cancer patients-Guideline on diagnosis and management by the Infectious Diseases Working Party of the German Society for Haematology and Medical Oncology. Eur J Cancer. 2016;67:200-212.
- Hirsch HH, Martino R, Ward KN, et al. Fourth European Conference on Infections in Leukaemia (ECIL-4): Guidelines for diagnosis and treatment of human respiratory syncytial virus, parainfluenza virus, metapneumovirus, rhinovirus, and coronavirus. Clin Infect Dis.2013;56(2):258-266.
- Choi SH, Hong SB, Ko GB, et al. Viral infection in patients with severe pneumonia requiring intensive care unit admission. Am J Respir Crit Care Med. 2012;186(4):325-332.
- van Someren Gréve F, Ong DS, Cremer OL, et al. Clinical practice of respiratory virus diagnostics in critically ill patients with a suspected pneumonia: A prospective observational study. J Clin Virol. 2016;83:37-42.
- Tregoning JS, Schwarze J. Respiratory viral infections in infants: Causes, clinical symptoms, virology, and immunology. Clin Microbiol Rev. 2010;23(1):74-98.
- Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095-128.
- Siegel JD, Rhinehart E, Jackson M, Chiarello L and the Healthcare Infection Control Practices Advisory Committee. Guideline for isolation precautions: preventing transmission of infectious agents in healthcare settings. February 2017. https://www.cdc.gov/infectioncontrol/guidelines/isolation/index.html.
- Centers for Disease Control and Prevention. DC Guidelines and recommendations: prevention strategies for seasonal influenza in healthcare settings. October 2016. https://www.cdc.gov/flu/professionals/infectioncontrol/healthcaresettings.htm.
- Krasinski K, LaCouture R, Holzman RS, et al. Screening for respiratory syncytial virus and assignment to a cohort at admission to reduce nosocomial transmission. J Pediatr. 1990;116(6):894-898.
- Centers for Disease Control and Prevention. Recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee. Guidelines for preventing health-care-associated pneumonia. March 2004. https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5303a1.htm.
- Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States. April 2017. http://www.cdc.gov/drugresistance/threat-report-2013/.
- Silverman M, Povitz M, Sontrop JM, et al. Antibiotic prescribing for non-bacterial acute upper respiratory infections in elderly persons. Ann Intern Med. 2017;166(11):765-774.
- Gonzales R, Malone DC, Maselli JH, Sande MA. Excessive antibiotic use for acute respiratory infections in the United States. Clin Infect Dis. 2001;33(6):757-762.
- Gill JM, Fleischut P, Haas S, et al. Use of antibiotics for adult upper respiratory infections in outpatient settings: A national ambulatory network study. Fam Med. 2006;38(5):349-354.
- Steinman MA, Landefeld CS, Gonzales R. Predictors of broad-spectrum antibiotic prescribing for acute respiratory tract infections in adult primary care. JAMA. 2003;289(6):719-725.
- Zoorob R, Sidani MA, Fremont RD, Kihlberg C. Antibiotic use in acute upper respiratory tract infections. Am Fam Physician. 2012;86(9):817-822.
- Hersh AL, Jackson MA, Hicks LA; American Academy of Pediatrics Committee on Infectious Diseases. Principles of judicious antibiotic prescribing for upper respiratory tract infections in pediatrics. Pediatrics. 2013;132(6):1146-1154.
- Centers for Disease Control and Prevention. National Strategy for Combating Antibiotic-Resistant Bacteria. September 2014. https://www.cdc.gov/drugresistance/pdf/carb_national_strategy.pdf.
- Centers for Disease Control and Prevention. Antimicrobial resistance: no action today, no cure tomorrow. April 2011. https://www.cdc.gov/media/releases/2011/f0407_antimicrobialresistance.html.
- Rogers BB, Shankar P, Jerris RC, et al. Impact of a rapid respiratory panel test on patient outcomes. Arch Pathol Lab Med. 2015;139(5):636-41.
- Rappo U, Schuetz AN, Jenkins SG, et al. Impact of early detection of respiratory viruses by multiplex PCR assay on clinical outcomes in adult patients. J Clin Microbiol. 2016;54(8):2096-20103.
- Martinez RM, Kay HE, Scicchitano LM, Wolk DM. Implementation of non-batched respiratory virus assay significantly impacts patient outcomes in the ICU. Poster presented at: The Clinical Virology Symposium; May 2016, Daytona Beach, Florida.
- Xu M, Qin X, Astion ML, et al. Implementation of FilmArray Respiratory Viral Panel in a core laboratory improves testing turnaround time and patient care. Am J Clin Pathol. 2013;139(1):118-123.
- Brendish NJ, Malachira AK, Armstrong L, et al. Routine molecular point-of-care testing for respiratory viruses in adults presenting to hospital with acute respiratory illness (ResPOC): A pragmatic, open-label, randomised controlled trial. Lancet Respir Med. 2017;5(5):401-411.
- American Society for Microbiology. Multiplex White Paper: Clinical utility of multiplex tests for respiratory and gastrointestinal pathogens. 2017. https://www.asm.org/index.php/statements-and-testimony/item/6691-wp-multiplex.
Stefan Juretschko, PhD, D(ABMM), serves as the Senior Director of the Division of Infectious Disease Diagnostics at the New York-based Northwell Health Laboratories. He oversees three hospital-based laboratories and a central Core Laboratory with full-service Clinical Microbiology, Mycology, Parasitology, Mycobacteriology, Virology and Molecular Diagnostics, supporting five tertiary, 12 community, three specialty, and two affiliated hospitals, along with more than 600 ambulatory and physician practices.