Hybrid power in laboratory instrumentation
Ongoing pressure to reduce healthcare costs, combined with a shortage of qualified histotechnicians, is driving testing labs to increase slide staining throughput and efficiency. Enhancing throughput is a multifactorial problem that weighs the tradeoffs among process speed, process reliability, and available equipment and labor, all with an overarching constraint that staining quality must be upheld. In the current challenging reimbursement climate it is essential that equipment manufacturers continually develop higher-throughput automated staining systems to improve diagnostics and, ultimately, patient care.
The first wave of fully automated slide stainers revolutionized the way in which clinical and research pathology labs organized their workflow, made staffing decisions, and reduced their sample turnaround time to meet increasing workloads. Automated staining instruments showcased the value of online deparaffinization and heat-induced epitope retrieval (HIER), leading to enhanced staining consistency in less time and with less labor. However, the first-wave instruments imposed a ceiling on the number of slides, typically 30, that could be processed in parallel even if more slides could be loaded into the machine.1,2
Once initial workflow improvements were realized with full automation, the parallel processing ceiling forced labs to deploy more instruments as a means of further increasing laboratory throughput. Labs without the space or budget for additional instruments could still increase overall throughput by performing deparaffinization and HIER of large numbers of slides offline. However, this strategy increased the labor burden and could fragment workflow due to the mismatch between high-capacity offline steps and lower-capacity stainers. Increasing numbers of labs, particularly those specializing in gastrointestinal, dermatological, and breast samples, require large-scale batching capacity to keep up with their constantly growing workload. As a result, full automation is no longer sufficient in and of itself. It is also imperative that manufacturers increase the number of slides that can be automatically processed in parallel.
The origin of the 30-slide parallel processing ceiling can ultimately be traced to the use of under-slide heaters for HIER. The poor thermal conductivity of the microscope slide itself, combined with the need to rapidly heat and hold temperature during HIER, necessitates the use of relatively powerful heaters that can exceed the power available from standard electrical circuits when more than 30 slides are in HIER at the same time. Under-slide heating also makes it difficult to determine the antigen retrieval (AR) solution temperature at the tissue level, limits the volume of AR solution (which leads to evaporation issues), and makes the heaters vulnerable to corrosion and failure due to the hostile under-slide environment.
Over time, manufacturers have minimized some of the issues associated with under-slide heating. For instance, evaporation can be controlled via the use of individual plastic cover tiles or liquid cover slips. Heater reliability has also been improved over time with better heater sealing techniques and improved fluid management. However, the fundamental issues of high power consumption and poor thermal control due to indirect tissue heating persist.
In order to achieve the goal of staining more than 30 slides in parallel and more slides per day, it is necessary to increase the amount of power available for the heating of AR solution from a standard wall outlet. Without costly and sometimes unavailable high-power dedicated circuits, the maximum power available to an instrument is typically ~1800 watts, comparable to that of a hair dryer.
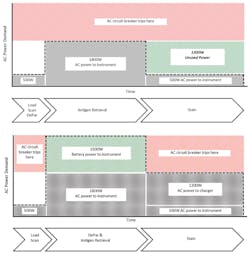
One strategy for increasing available HIER power relies on a common characteristic of most staining protocols for formalin fixed paraffin embedded (FFPE) tissues. The power-intensive HIER phase of FFPE tissue staining constitutes less than one-half the total slide processing time. Later protocol phases tend to require much less power since the slides are stained at room temperature. A high-capacity energy storage device (e.g., a lithium ion battery) can be used to boost the power available during the HIER phase, after which the storage device is recharged during the remainder of the staining protocol when excess power is available from the wall circuit. Such a strategy relies on a specialized device called an inverter/charger that converts the battery’s direct current (DC) power to AC power and combines it with AC power from the wall circuit, thereby increasing the power available from ~1800W to over 3000W. Though inverter/chargers are not generally employed in biomedical or other instrumentation, they are well-proven and widely used in high reliability grid-tied and battery-backed power installations that rely on transient charging via solar panels and/or generators. Using such a strategy with a modestly-sized lithium battery, parallel slide processing capacity can be increased from 30 slides to 48 slides, a 60 percent increase (Figure 1).
The advent of fully automated stainers transformed the workflow of large and small labs alike and made automated immunohistochemistry, immunofluorescence, and in situ hybridization routine. Such developments have allowed labs to adopt more staining techniques and produce more consistent results with limited staff, thereby saving time and cost while improving patient care. As medicine and molecular diagnostics become increasingly more sophisticated, the throughput and performance of slide staining instruments will become critical. Hybrid power systems incorporated into the next generation of instruments will therefore become an important enabler for laboratories going forward.
REFERENCES
- Prichard JW. Overview of automated immunohistochemistry. Arch Pathol Lab Med. 2014;138 (12):1578-1582.
- Aller RD, Weiner H, DeSalvo W. Automated staining instruments. CAP TODAY. 2017; 31(8):40-49.
Jennifer Schwedler, PhD, serves as Technical Writer and Consultant for California-based Biocare Medical.
David Basiji, PhD, serves as Vice President of Engineering for Biocare Medical.