Overcoming the challenges of genital lesion diagnostics using multiplex molecular testing
There are a number of infectious agents that cause ulceration or lesions in the genital area, and although many may be considered sexually transmitted diseases (STDs), etiology is complex, with similarities in symptomology that calls for the use of appropriate diagnostics to cover a range of possibilities. Table 1 outlines the estimated incidences of what can be considered the main causes of genital, anal, or perianal ulcers and lesions, with herpes simplex viruses (HSV) by far the most common etiological agent.1,2 It is difficult to ascertain the true prevalence of HSV, as the majority of infections are asymptomatic; however, data indicate a higher incidence of HSV-2 genital infections,2 with an increasing trend of HSV-1 genital infections occurring worldwide.3,4
The second most likely cause is syphilis infection with the bacterium Treponema pallidum.1 This past decade has seen an increase in syphilis infection rates, most notable in high-risk populations such as men who have sex with men (MSM).5,6 Recent U.S. data showing increases of 19 percent in primary and secondary syphilis across MSM and neonatal infections have prompted the U.S. Centers for Disease Control and Prevention (CDC) to draft a call to action to “Stem the Tide of Rising Syphilis in the United States,” wherein the agency recognizes “a critical need for commercially available direct detection tests.”7
The CDC also lists chancroid infection with the pathogen Haemophilus ducreyi as a notable cause of genital lesions.1 Although still relevant in some areas of Africa and the Caribbean, chancroid is rare in the United States and is declining worldwide.1,8 Some of the other less-common causes noted are lymphogranuloma venereum (LGV) caused by specific serotypes of Chlamydia trachomatis, still determined to be rare, but with sporadic outbreaks increasingly reported,1,9 and granuloma inguinale (donovanosis), another rarely encountered infection in the U.S. caused by Klebsiella granulomatis.1 Included in Table 1 is another herpes virus, varicella zoster (VZV), not typically associated with genital infection. VZV can present as ulcers in the genital region, with a recent study uncovering 11 percent incidence in genital lesions while concurrently testing for HSV infection.10
Diagnosis and management of genital lesions
With such a wide range of causative agents manifesting as genital ulceration, diagnosis based purely on symptomology and risk factors can be difficult and is discouraged.1 Each of the infectious agents described above requires different approaches to patient management. Even within HSV infection, prognosis varies between HSV-1 and -2, with the latter most likely to cause recurrent disease.11 In the case of VZV, in one study misdiagnosis has been found in up to 20 percent of patients with initial diagnosis of HSV infection.12 Implications of VZV misdiagnosis can range from psychosocial to legal, such as in one misreported pediatric example leading to an incorrect charge of suspected child abuse.13 Atypical presentation of VZV infection usually occurs in immunocompromised patients;14 however, reactivation of VZV infections results in herpes zoster, which can more commonly occur in unusual anatomical sites,10,12 and is important to accurately diagnose in order to treat early and avoid complications of neuralgia.
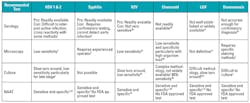
Table 2 lists the range of diagnostic approaches recomended by the CDC, along with associated pros and cons. Of the recommended approaches, including serology, culture, and nucleic acid amplification testing (NAAT), only NAATs can offer high enough specificity and sensitivity to be suitable as a definitive diagnostic in most scenarios. There are limited commercial NAAT options for genital lesion pathogens, with only a few FDA-approved HSV NAATs, and there is no single FDA-approved diagnostic that covers all pathogens. Serology and culture are often favored in diagnostic laboratories due to their relatively low cost and ease of use, but significant false positive and negative results occur, requiring mandatory confirmatory testing,1 and early infections will not be detected.
For HSV diagnostics, NAATs are now considered the standard,15 and with the dramatic increase in syphilis the CDC is also calling for direct syphilis tests, such as NAATs, to overcome the limitations of current serological tests.7 Dark-field microscopy, once considered the diagnostic standard for syphilis, requires skilled and experienced operators, and laboratories worldwide are increasingly turning to NAATs for diagnosis of primary and secondary infections. The process of validating an in vitro diagnostic (IVD) for rare conditions such as chancroid or LGV would be complex due to the scarcity of positive clinical specimens. A multiplex NAAT that included the most prevalent infectious candidates—HSV-1, HSV-2, syphilis, and VZV—would be an ideal solution. However, combining multiple targets into multiplex molecular tests is not without its challenges.
Overcoming multiplex molecular testing challenges
Traditional qPCR probe methodologies used extensively in IVD, although sensitive and highly specific, have well-known limitations when it comes to multiplexing, including non-specific amplification and sacrificed sensitivity.18,19 There are many examples, both commercially available or described in peer-reviewed literature, of duplex and triplex qPCR reactions; however, protocols involving ≥4 targets are rare, typically requiring complex and costly optimization of primer and probe combinations.20,21 There have been a number of attempts to overcome these limitations, typically requiring specialized equipment,22 multiple reactions,23 or other techniques that increase the barrier for adoption in a cost- and time-constrained clinical lab.
One novel approach is compatible with existing laboratory instrumentation, and utilizes target-specific DNA enzymes designed to cleave a suite of well-characterized proprietary reporter probes. The de-coupling of the probe design from target sequences facilitates their application in highly specific and sensitive multiplex qPCR.24 This approach resembles traditional qPCR, in that target-specific primers are required for amplification; however, detection of the resulting amplicon does not require sequence-specific probes, and thus reaction complexity is minimized, reducing the risk of competitive binding events and maintaining assay specificity and sensitivity. The two-part structure provides high-level specificity when binding to the target amplicon; universal probes then recognize the active enzymes, resulting in enzymatic cleavage that generates fluorescence to be read in real time.24 Examples of quadruplex and quintuplex versions of this approach have been shown to maintain the same sensitivity and specificity of single PCR comparisons,24 and this approach has been utilized in a number of CE-IVD multiplex diagnostic tests.25
A development assay utilizing this approach to combine detection of HSV-1, HSV-2, syphilis, VZV, and an amplification/internal control has been evaluated by Westmead Hospital (Sydney, Australia) (currently not available in the U.S.). Results were presented at the World STI Congress in Rio de Janeiro26 using retrospective clinical samples to compare sensitivity and specificity of the five-plex multiplex with existing in-house methods. Results demonstrate that the new technology can maintain high levels of sensitivity and specificity in five target multiplex applications, allowing for the development of NAATs to meet the broad target requirements of genital lesion diagnostics and other complex syndromic disease states.
A Clinician’s View
“As a doctor in a UK sexual health clinic we have seen a large rise in people attending with possible acute syphilis, with genital, anal and mouth ulceration or proctitis. We are lucky to have skills in the clinic for dark-ground microscopy but this is only available in one location and depends on a single skilled operator. Having access to routinely multiplexed PCR testing for herpes type 1 & 2 and T. pallidumallows us to make a confident diagnosis of early syphilis in a community setting or elsewhere in the hospital. A positive PCR can also help date the infection and limit the look-back time for partner notification. We strongly believe in having the T. pallidum PCR as part of the routine test for any genito-anal ulcer. Several patients have been found to have syphilis where this was not originally suspected, and blood tests would never have been taken. Syphilis can have very serious and occasionally life-threatening complications which are avoided completely by early diagnosis and treatment.”
—Dr. Andrew Winter,
Consultant in Sexual Health and HIV Medicine,
NHS Greater Glasgow and Clyde, UK
REFERENCES
- Workowski KA, Bolan GA, Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64 (RR03);25-48
- Looker KJ, Magaret AS, Turner KME, et al. Global and regional estimates of prevalent and incident herpes simplex virus type 2 infections in 2012. PLoS ONE. 2015;10(1): e114989.
- Peña C, Adelson M, Mordechai E, Blaho J. Genital herpes simplex virus type 1 in women: detection in cervicovaginal specimens from gynecological practices in the United States. J Clin Microbiol. 2010;48(1):150–153
- Ashley R, Wald A. Genital herpes: review of the epidemic and potential use of type-specific serology. Clin Microbiol Rev. 1999; 12(1):1–8
- European Centre for Disease Prevention and Control. Syphilis—annual epidemiological report 2016 [2014 data]. https://ecdc.europa.eu/en/publications-data/syphilis-annual-epidemiological-report-2016-2014-data Published April 20, 2016. Accessed August 23, 2017.
- Centers for Disease Control and Prevention. Syphilis & MSM. (Fact Sheet). https://www.cdc.gov/std/syphilis/stdfact-msm-syphilis.htm.
- Centers for Disease Control and Prevention. CDC call to action: Let’s work together to stem the tide of rising syphilis in the United States. https://www.cdc.gov/std/syphilis/syphiliscalltoactionapril2017.pdf
- González-Beiras C, Marks M, Chen CY, Roberts S, Mitjà O. Epidemiology of Haemophilus ducreyi infections. Emerging Infectious Diseases. 2016;22(1):1-8.
- Ceovic R, Gulin SJ. Lymphogranuloma venereum: diagnostic and treatment challenges. Infection and Drug Resistance. 2015;8:39-47.
- Granato PA, DeGilio MA, Wilson EM. The unexpected detection of varicella-zoster virus in genital specimens using the Lyra™ Direct HSV 1+2/VZV Assay. J Clin Virol.2016;84:87-89
- Lafferty WE, Downey L, Celum C, Wald A. Herpes simplex virus type 1 as a cause of genital herpes: impact on surveillance and prevention. J Infect Dis. 2000;181(4):1454-1457.
- Rubben A, Baron JM, Grussendorf-Conen El. Routine detection of herpes simplex virus and varicella zoster virus by polymerase chain reaction reveals that initial herpes zoster is frequently misdiagnosed as herpes simplex, Br. J Dermatol. 1997;137(2):259–261.
- Christian CW, Singer ML, Crawford JE, Durbin D. Perianal herpes zoster presenting as suspected child abuse. J Pediatr. 1997;99(4):608-610.
- Pillet S, Verhoeven PO, Epercieus A, Bourlet T, Pozzello B. Development and validation of a laboratory-developed multiplex real-time PCR assay on the BD Max system for detection of herpes simplex virus and varicella-zoster Virus DNA in various clinical specimens. J Clin Microbiol. 2015j;53(6):1921-1926.
- Sauerbrei A. Optimal management of genital herpes: current perspectives. Infect Drug Resist. 2016;9:129–141.
- Gayet-Ageron A, Sednaoui P, Lautenschlager S, et al. Use of Treponema pallidum PCR in Testing of Ulcers for Diagnosis of Primary Syphilis. Emerg Infect Dis. 2015;21(1):127-129.
- Gayet-Ageron A, Lautenschlager S, Ninet B, Perneger TV, Combescure C. Sensitivity, specificity and likelihood ratios of PCR in the diagnosis of syphilis: a systematic review and meta-analysis. Sex Transm Infect. 2013;89:251-6
- Wang X, Theodore MJ, Mair R, et al. Clinical validation of multiplex real-time PCR assays for detection of bacterial meningitis pathogens. J Clin Microbiol. 2012;50(3):702-708.
- Gunson RN, Bennett S, Maclean A, Carman WF. Using multiplex real time PCR in order to streamline a routine diagnostic service. J Clin Virol. 2008;43(4)372–375.
- Kirchner S, Krämer KM, Schulze M, et al. Pentaplexed quantitative real-time PCR assay for the simultaneous detection and quantification of botulinum neurotoxin-producing clostridia in food and clinical samples. Appl Environ Microbiol. 2010;76:4387–4395.
- Murphy J, Bustin SA. Reliability of real-time reverse-transcription PCR in clinical diagnostics: gold standard or substandard? Expert Rev Mol Diagn. 2009;9(2):187-197.
- Poritz MA, Blaschke AJ, Byington CL, et al. FilmArray, an automated nested multiplex pcr system for multi-pathogen detection: development and application to respiratory tract infection. Costa C, ed. PLoS ONE. 2011;6(10):e26047. doi:10.1371/journal.pone.0026047.
- Richmond GR, Khine H, Zhou TT, et al. MassCode liquid arrays as a tool for multiplexed high-throughput genetic profiling.PLoS One. 2011;6(4):e18967.
- Mokany E, Tan YL, Bone SM, Fuery CJ, Todd AV. MNAzyme qPCR with superior multiplexing capacity. Clin Chem. 2013;59(2):419-426.
- Tabrizi SN, Tan LY, Walker S, et. al. Multiplex assay for simultaneous detection of Mycoplasma genitalium macrolide resistance using PlexZyme and PlexPrime technology. PLoS One. http://journals.plos.org/plosone/article?id=10.1371/journal.pone.01567402016.
- Vipond B, Green N, Longhurst D et. al. SPEEDX PLEXPCR™ VHS EVALUATION. Poster: IUSTI 2017.
- Windsor M, Njuguna P, Tucker R, et.al. Multiplex assay for the detection of syphilis and other pathogens associated with genital lesions using PlexPCRTM. Poster: IUSTI World 2017.
- Looker KJ, Magaret AS, Kay MT et. al. 2015 Global and regional estimates of prevalent and incident herpes simplex virus type 1 infections in 2012. PLoS One. 10(10):e0140765.
- Newman L, Rowley J, Vander Hoorn S, et al 2015. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS One. 10(12):e0143304
- Reynolds MA, Kruszon-Moran D, Jumaan A, Schmid DS, McQuillan GM. Varicella seroprevalence in the U.S.: Data from the National Health and Nutrition Examination Survey, 1999–2004. Public Health Reports. 2010;125(6):860-869.
- Lopez AS, Zhang J, Marin M. Epidemiology of varicella during the 2-Dose Varicella Vaccination Program—United States, 2005–2014. MMWR. 2016;65:902–905.
- European Centre for Disease Prevention and Control. Varicella vaccine in the European Union Stockholm: ECDC; 2014.
- Seward J, Jumaan A. VSV: persistence in the population. In: Arvin A, Campadelli-Fiume G, Mocarski E, et al., editors. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge: Cambridge University Press; 2007. Chapter 40. https://www.ncbi.nlm.nih.gov/books/NBK47367/
- Lee BW. 1998. Review of varicella zoster seroepidemiology in India and South East Asia. Tropical Medicine and International Health. 1998.3(11):886-890.
- O’Farrell N. Tropical Medicine Series. Donovanosis. Sex Transm Infect. 2002;78:452–457
- World Health Organisation 2012. Strategies and laboratory methods for strengthening surveillance of sexually transmitted infection. 2012 UNAIDS/WHO Working Group on Global HIV/AIDS and STI Surveillance.
- Kawai K, Gebremeskel B, Acosta C. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;4(6), pp.e004833-e004833.
- Riera-Montes M, Bollaerts K, Heininger U, et al. Estimation of the burden of varicella in Europe before the introduction of universal childhood immunization. BMC Infectious Diseases 2017;17:353.
- Breuer J, Schmid DS, Gershon AA. Use and limitations of varicella-zoster virus-specific serological testing to evaluate breakthrough disease in vaccinees and to screen for susceptibility to varicella. J Infect Dis. 2008;197 Suppl 2:S147
- Alfa M. The laboratory diagnosis of Haemophilus ducreyi. Can. J Infect Dis Med Microbiol. 2005;16(1):31-34.
- Papp JR, Schachter J, Gaydos CA, et al. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae—2014. MMWR Recommendations and Reports. March 14, 2014 / 63(RR02);1-19
- Harwood-Nuss A. Chapter 272: Rashes: Vesicles, Pustular and Scaly Lesions. In: Wolfsen AB, ed. Harwood-Nuss’ Clinical Practice of Emergency Medicine. Philadelphia, PA: Lippincott Williams & Wilkins.; 2001:1321
- Gnann JW Jr, Whitley RJ Clinical practice. Herpes zoster. N Engl J Med. 2002;347(5):340.
- Schmutzhard J, Merete Riedel H, Zweygberg Wirgart B, Grillner L. Detection of herpes simplex virus type 1, herpes simplex virus type 2 and varicella-zoster virus in skin lesions. Comparison of real-time PCR, nested PCR and virus isolation. J Clin Virol. 2004;29(2):120.
Elisa Mokany, PhD, is co-founder and Chief Technology Officer at Sydney, Australia-based SpeeDx. She leads the development of diagnostic technologies and commercialization of infectious disease and antibiotic resistance tests. She has 17 years of experience in medical research, and is an inventor on eight patents/patent applications involving nucleic acid analysis including the novel PlexPrime technology, which has multiple applications in the in vitro diagnostic field.