Clinical and diagnostic challenges of antimicrobial resistance in Mycoplasma genitalium
CONTINUING EDUCATION
To earn CEUs, visit www.mlo-online.com under the CE Tests tab.LEARNING OBJECTIVES
1. Discuss the history, progression, symptoms, and disease states of M. genitalium.
2. Identify risk factors for M. genitalium and describe the concerns with current treatment regimens.
3. Discuss current testing methods for M. genitalium and describe the limitations associated with each one.
4. List and describe alternative testing methods and treatment options for M. genitalium infection.
Mycoplasma genitalium is a small bacterium from the Mollicute class that was first isolated from the human urogenital tract in the 1980s.1 It has taken some time to gain traction as an accepted sexually transmitted infection (STI); however, the U.S. Centers for Disease Control and Prevention’s (CDC) most recent guidelines for sexually transmitted diseases (STDs)2 have highlighted M. genitalium as an emerging issue. M. genitalium has been strongly and consistently associated with non-gonococcal urethritis (NGU) and cervicitis, and is also implicated in pelvic inflammatory disease in women.3-6
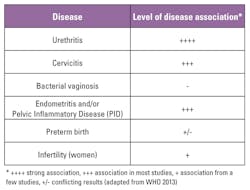
Table 1 summarizes the diseases associated with M. genitalium and the level of evidence for this association, as published in the World Health Organization (WHO) manual, Laboratory Diagnosis of Sexually Transmitted Infections, Including Human Immunodeficiency Virus.5
Disease in men
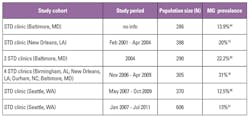
M. genitalium was first discovered in men with NGU, and most of the subsequent studies focus in the same manner.4 Urethritis is an inflammation of the urethra and may be characterized by symptoms such as dysuria, urethral pruritus, and purulent or non-purulent urethral discharge. Urethritis can have many infectious etiologies. When it is not caused by a gonorrheal infection it is known as non-gonococcal urethritis or NGU. C. trachomatis is the primary cause of NGU, responsible for 15 percent to 40 percent of cases,7 and M. genitalium is now recognized as the second most common cause, responsible for 15 percent to 25 percent of cases in men with symptomatic NGU.4 U.S. studies on M. genitalium prevalence in men with NGU range from 13 percent to 31 percent. (Table 2)
The strong association of M. genitalium with NGU has led to its inclusion in diagnostic considerations by the Centers for Disease Control and Prevention’s Sexually Transmitted Diseases Treatment Guidelines,2 European guidelines for management of NGU,8 and the European guideline on Mycoplasma genitalium infections.9 M. genitalium infection is also strongly correlated with persistent or recurrent NGU, found in 19 percent to 41 percent of men with persistent or recurrent disease.4,10,11 These high levels may be a consequence of current treatment practices resulting in inadequate syndromic treatment of NGU.
Disease in women
Mucopurulent cervicitis has also been described as the female counterpart of NGU in men,8 and non-gonococcal mucopurulent cervicitis is caused by similar etiologic agents. C. trachomatis is responsible for 20 percent to 40 percent of cases, and M. genitalium is responsible for five percent to 20 percent of cases.8
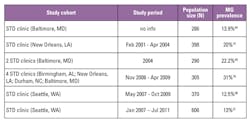
Meta-analysis studies on the disease association of M. genitalium infection and female reproductive tract syndromes have shown significant association with increased risk of cervicitis, pelvic inflammatory disease (PID), pre-term birth, spontaneous abortion, and risk of infertility.1,6 U.S. studies on M. genitalium prevalence in women with cervicitis range from 11.2 percent to 28.6 percent. (Table 3)
Prevalence of M. genitalium
From the limited data available, the prevalence of M. genitalium in the general population is reported at between 1.1 percent and 3.3 percent. In comparison to other causes of sexually transmitted infections, this is higher than the prevalence of Neisseria gonorrhoeae at 0.4 percent, but lower than Chlamydia trachomatis at 4.2 percent and Trichomonas vaginalis at 2.3 percent.4,2 Similar to C. trachomatis, M. genitalium can also be found in asymptomatic infection12,13; thus the true prevalence may be underestimated.
A recent multicenter clinical study cohort in the U.S. investigated the prevalence of M. genitalium infections in urogenital specimens collected from 946 male and female subjects.14 M. genitalium prevalence rates were 16.1 percent for females and 17.2 percent for males. Significant risk factors for M. genitalium infections were African descent, younger age, non-Hispanic ethnicity, and female symptomatic status.
Management of NGU and cervicitis
Different etiologic agents, such as C. trachomatis and M. genitalium, can have similar clinical presentations, as seen in urethritis and cervicitis. Therefore these STIs are commonly managed as syndromes grouped by similar symptoms. In syndromic management, treatment is targeted at the most common causes at the time of diagnosis and before the etiologic agent is identified.
Urethritis in symptomatic men is first determined to be gonococcal or non-gonococcal using microscopy. In the U.S., the Sexually Transmitted Diseases Treatment Guidelines, 2015, recommend presumptive treatment of NGU with doxycycline, a tetracycline antibiotic, or azithromycin, a macrolide antibiotic.2 Doxycycline and azithromycin are highly effective against C. trachomatis (67 percent to 87 percent for men with NGU); however, doxycycline regimens have poor cure rates for M. genitalium (30 percent to 45 percent).15,16
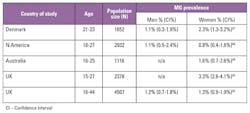
Therefore, if doxycycline was given as the first line therapy for NGU where M. genitalium was the causative agent, treatment is more likely to fail. Of further concern is the high rate of M. genitalium macrolide resistance appearing in studies world-wide.14,17-29,31 The most recent and comprehensive U.S. study found macrolide-resistant M. genitalium phenotypes in 51 percent of females and 42 percent of males,14 indicating that a significant portion of the tested population would not respond to current frontline treatment recommendations.
The practice of syndromic treatment and subsequent use of doxycycline and/or azithromycin without appropriate and targeted diagnostics is likely contributing to the high prevalence of M. genitalium in persistent or recurrent urethritis.
Currently available tests for M. genitalium
Although M. genitalium has been in the literature for several decades, it has only recently been more broadly recognized as an STI1 and is currently not listed as a notifiable disease; thus, testing patterns vary widely throughout the U.S. and globally. Whereas both chlamydia and gonorrhea are widely tested and reported on, there are no official guidelines on screening for M. genitalium in sexually active individuals, and many clinics do not currently request testing for this pathogen in initial consultations. More recent publications and discussions are now calling for the monitoring of M. genitalium in high-risk populations.14,27-29
When testing does occur, laboratories have limited options. M. genitalium is a fastidious organism and grows very slowly in culture, taking up to six months to detect.1 Only a few labs globally have been able to isolate M. genitalium from clinical samples. Additionally, due to closely related species, serology lacks adequate specificity.30 Therefore, culture and serology, which are the traditional diagnostic methods for bacteria, are not suitable for routine testing of M. genitalium. The only viable diagnostic option is detection using a nucleic acid amplification test (NAAT).
There are currently no U.S. Food and Drug Administration (FDA)-cleared tests for detection of M. genitalium. For that reason, in-house NAATs have been extensively used along with available CE-marked or research use only (RUO) assays. The majority of in-use tests allow only for detection of M. genitalium, providing no information on potential resistance status of an infection.29 The rise in prevalence of antibiotic-resistant strains, however, has influenced the latest European guidelines for the management of M. genitalium infection, recommending the addition of resistance testing for effective management of NGU and other M. genitalium infections.8,9
Mutations associated with azithromycin resistance
Macrolide resistance in M. genitalium has been strongly and consistently associated with mutations occurring at positions 2058 and 2059 (Escherichia coli numbering) of region V of the 23S rRNA gene.4,32 The five most common mutations are A2058G (adenine transition to guanine base at position 2058), A2059G, A2058T, A2058C, and A2059C,32 and those are the only mutations found in the majority of studies.14,17-27 Isolated M. genitalium strains with four of the most common mutations have been shown to confer high-level azithromycin resistance in vitro,33 and all five mutations have been found in samples from patients exhibiting azithromycin treatment failure.4
There have been limited U.S. studies investigating 23S rRNA mutation prevalence. One study investigating M. genitalium prevalence also analyzed a subset of subjects for macrolide-resistant mutations and found rates of 50.8 percent [95 percent CI, 42.2 percent to 59.3 percent] in females (65 of 128) and 42 percent [95 percent CI, 29.4 percent to 55.8 percent] in males (21 of 50), with an overall prevalence of 48.3 percent.14
Testing for antibiotic resistance in M. genitalium
Given the high failure rates of the now standard azithromycin treatment, a detection-only assay for M. genitalium may have limited use in informing effective patient management. The European guidelines for management of NGU8 now recommend screening of male patients with urethritis for macrolide resistance mutations in order to improve clinical outcomes.
There are currently no FDA-cleared tests for detection of M. genitalium or for the determination of resistance status. With resistance linked to multiple mutations in the 23S rRNA gene, many studies rely on sequencing methods to determine resistant phenotypes14,27 This approach is not readily conducive to clinical practice due to high cost and increased sample processing times. Some alternative in-house approaches include the use of high resolution melting (HRM)34 or qPCR based on fluorescence resonance emission transfer (FRET) coupled with melting curve analysis.20 Clinical practices rarely incorporate HRM methodologies, as results may vary due to DNA concentration and can be difficult to reproduce in other labs, and melt curve analysis requires skilled interpretation. The example of the FRET assay is also not suitable for clinical purposes, as researchers only achieved a sensitivity of 76.7 percent.20
NAAT diagnostics for allelic discrimination typically utilize qPCR with allele-specific primers or detection with allele-specific fluorescent probes.35 This approach works well for conditions involving a single nucleotide polymorphism (SNP); however, design costs and complexity increase when multiple SNPs are involved. Often there are multiple variations within the same location, and/or SNPs are clustered within areas associated with specific structural or functional importance, leading to difficulties with cross priming or primer competition that affects specificity and sensitivity of the assay.36 The mutations associated with azithromycin resistance in M. genitalium represent such complexity.
The one available CE marked test for both detection of M. genitalium and resistance markers incorporates a novel priming technology to overcome these diagnostic challenges. This technology introduces an insert sequence during amplification, creating amplicons that are distinctly different from the original sequence.36 Advantages to this approach may include increased stringency and subsequent selective amplification of mutant alleles, as well as the ability to multiplex a number of different SNP targets.36 The assay combines detection of M. genitalium with five azithromycin-associated mutations, multiplexing the detection of multiple resistance markers within a single-well reaction.37 Tested on 400 stored clinical samples of positive M. genitalium patients, M. genitalium detection sensitivity was 99.1 percent, specificity 98.5 percent, while sensitivity and specificity of mutation detection was 97.4 percent and 100 percent respectively.37
Treatment of M. genitalium
Mycoplasma lack a cell wall and thus are unaffected by many common antibiotics, leaving limited treatment options. Although the CDC STD guidelines recommend either doxycycline or azithromycin for the presumptive treatment of NGU,2 due to the rising recognition of M. genitalium in NGU, and the known ineffectiveness of doxycycline, many countries (Australia, New Zealand, United Kingdom, Denmark, Japan) use only azithromycin.21 The European guidelines on Mycoplasma genitalium infections9 specifically recommend azithromycin for treatment of uncomplicated M. genitalium infections.
While azithromycin is more effective than doxycycline in the treatment of M. genitalium, cure rates have been declining. Three clinical trials across a decade in the U.S. showed declining cure rates from 77 percent in 2002-200415 to 67 percent in 2006-200916 to 40 percent in 2007-2011.31 Lau et al38 recently published a meta-analysis of 21 studies on the efficacy of azithromycin for M. genitalium treatment, demonstrating declining cure rates since 2009. The pooled microbial cure for the 12 studies conducted prior to 2009 was 85.3 percent (95 percent confidence interval [CI], 82.3 percent to 88.3 percent). For the nine studies conducted since the beginning of 2009, the pooled microbial cure was 67.0 percent (CI, 57.0 percent to 76.9 percent).38
M. genitalium treatment failures can be addressed with moxifloxacin. Initial reports showed moxifloxacin to be highly effective,1,11 yet its use is limited due to cost, side effects, availability, and potential for development of resistance. Resistance to moxifloxacin has been observed over the last decade, often in conjunction with macrolide resistance, resulting in persistent infection that is very challenging to manage.29
M. genitalium is exhibiting alarming capabilities of developing antimicrobial resistance (AMR), and the widespread use of azithromycin as front-line treatment appears to be driving even higher rates of resistance.28,29,38 There is a real clinical need to accompany M. genitalium testing with surveillance of macrolide resistance-mediating mutations to enhance patient management and control the spread of AMR.8,29,37,39,40 Dr. Margaret Chan, the WHO director, summarized perfectly the larger AMR issue facing us when she said, “Today, antibiotics are rarely prescribed based on a definitive diagnosis. Having rapid, low-cost, and readily available diagnostics is an essential part of the solution to this urgent problem.”50
REFERENCES
- Tully JG, Taylor-Robinson D, Cole RM, Rose DL. A newly discovered mycoplasma in the human urogenital tract. Lancet. 1981;I:1288–1291.
- Workowski K, Bolan G. Sexually Transmitted Diseases Treatment Guidelines, 2015. MMWR Recomm Rep 2015;64(3):1-135.
- Jensen JS, Bjornelius E, Dohn B, Lidbrink P. Use of TaqMan 5’ nuclease real-time PCR for quantitative detection of Mycoplasma genitalium DNA in males with and without urethritis who were attendees at a sexually transmitted disease clinic. J Clin Microbiol. 2004;42(2): 683-692.
- Taylor-Robinson D, Jensen JS. Mycoplasma genitalium: from chrysalis to multicolored butterfly. Clin Microbiol Rev. 2011;24(3):498–514.
- World Health Organization (WHO). Laboratory diagnosis of sexually transmitted infections, including human immunodeficiency virus. Switzerland: World Health Organization 2013.
- Lis R, Rowhani-Rahbar A, Manhart LE. Mycoplasma genitalium infection and female reproductive tract disease: a meta-analysis. Clin Infect Dis. 2015;61(3):418-426.
- Centers for Disease Control and Prevention (CDC). Diseases characterized by urethritis and cervicitis in Sexually Transmitted Diseases Guidelines. 2015. http://www.cdc.gov/std/tg2015/urethritis-and-cervicitis.htm.
- Horner PJ, Blee K, Falk L, vander Meijden W, Moi H. 2016 European Guideline on the management of non-gonococcal urethritis. Int J STD AIDS. 2016;27(11):928-937.
- Jensen JS, Cusini M, Gomberg M, Moi H. 2016 European guideline on Mycoplasma genitalium infections. J Eur Acad Dermatol Venereol. 2016;30(10):1650-1656.
- Wikstrom A, Jensen JS. Mycoplasma genitalium: a common cause of persistent urethritis among men treated with doxycycline. Sex Transm Infect. 2006; 82(4):276–279.
- Manhart LE, Broad JM, Golden MR. Mycoplasma genitalium: should we treat and how? Clinical Infectious Diseases. 2011;53(Suppl 3):S129-S142.
- Falk L, Fredlund H, Jensen, JS. Signs and symptoms of urethritis and cervicitis among women with or without Mycoplasma genitalium or Chlamydia trachomatis infection. Sexually Transmitted Infections. 2005;81(1):73–78.
- Ross JD, C., Brown L, Saunders P, Alexander S. Mycoplasma genitalium in asymptomatic patients: implications for screening. Sexually Transmitted Infections. 2009; 85(6):436–437.
- Getman D, Jiang A, O’Donnell M, Cohen S. Mycoplasma genitalium prevalence, coinfection, and macrolide antibiotic resistance frequency in a multicenter clinical study Cohort in the United States. (R. Patel, Ed.). J Clin Microbiol. 2016;54(9):2278–83.
- Mena LA, Mroczkowski TF, Nsuami M, Martin DH. A randomized comparison of azithromycin and doxycycline for the treatment of Mycoplasma genitalium-positive urethritis in men. Clin Infect Dis. 2009;48(12):1649–1654.
- Schwebke JR, Rompalo A, Taylor S, et al. Re-evaluating the treatment of nongonococcal urethritis: Emphasizing emerging pathogens – A randomized clinical trial. Clin Infect Dis. 2011;52(2):163–170.
- Tagg KA, Jeoffreys NJ, Couldwell DL., Donald JA, Gilbert GL. Fluoroquinolone and macrolide resistance-associated mutations in Mycoplasma genitalium. J Clin Microbiol. 2013;51(7):2245–2249.
- Gesink DC, Mulvad G, Montgomery-Andersen R. Presence, resistance and epidemiology in Greenland. International Journal of Circumpolar Health. 2012;71(0):1–8.
- Pond MJ, Nori AV Witney AA, et al. High prevalence of antibiotic-resistant Mycoplasma genitalium in nongonococcal urethritis: the need for routine testing and the inadequacy of current treatment options. Clin Infect Dis. 2014;58(5):631–637.
- Touati A, Peuchant O, Jensen JS, Bébéar C, Pereyrea S. Direct detection of macrolide resistance in Mycoplasma genitalium isolates from clinical specimens from France by use of real-time PCR and melting curve analysis. J Clin Microbiol. 2014;52(5):1529–1555.
- Bissessor M, Tabrizi SN, Twin J, et al. Macrolide resistance and azithromycin failure in a Mycoplasma genitalium-infected cohort and response of azithromycin failures to alternative antibiotic regimens. Clin Infect Dis. 2015;60(8):1228–1236.
- Kikuchi M, Ito S, Yasuda M, et al. Remarkable increase in fluoroquinolone-resistant Mycoplasma genitalium in Japan. J Antimicrob Chemother. 2014;69(9):2376–2382.
- Gossé M, Nordbø SA, Pukstad B. Bacterial load in daily urine samples of atients infected with Mycoplasma genitalium, mutation analysis, and response to treatment. Infectious Diseases in Obstetrics and Gynecology. 2016;.doi.org/10.1155/2016/8382469.
- Kristiansen GQ, Lisby JG, Schønning K. A 5’ nuclease genotyping assay for identification of macrolide-resistant Mycoplasma genitalium in clinical specimens. J Clin Microbiol. 2016;54(6):1593-1597.
- Dumke R, Thürmer A, Jacobs E. Emergence of Mycoplasma genitalium strains showing mutations associated with macrolide and fluoroquinolone resistance in the region Dresden, Germany. Diagn Microbiol Infect Dis. 2016;86(2):221–223.
- Chernesky M, Jang D, Gilchrist J, et al. Mycoplasma Genitalium (MG) Infections in Canadian Women with Chlamydia Trachomatis (CT) and/or Neisseria Gonorrhoeae (NG). Poster presented at 2016 CDC STD Prevention Conference.
- Anagrius C, Lore B, Jensen JS. Treatment of Mycoplasma genitalium. Observations from a Swedish STD clinic. PloS One. 2013 8(4):e61481.
- Jensen JS, Bradshaw C. Management of Mycoplasma genitalium infections—can we hit a moving target? BMC Infectious Diseases. 2015;15(1):343.
- Unemo M, Jensen JS. Antimicrobial-resistant sexually transmitted infections: gonorrhoea and Mycoplasma genitalium. Nature Reviews Urology. 2017;14(3):139-152.
- Lind K, Lindhardt BO, Schütten HJ, Blom J, Christiansen C. Serological cross-reactions between Mycoplasma genitalium and Mycoplasma pneumoniae. J Clin Microbiol. 1984; 20(6):1036–1043.
- Manhart LE, Gillespie CW, Lowens MS, et al. Standard treatment regimens for nongonococcal urethritis have similar but declining cure rates: A randomized controlled trial. Clin Infect Dis. 2013;56(7):934–942.
- Couldwell DL, Tagg KA, Jeoffreys NJ, Gilbert GL. Failure of moxifloxacin treatment in Mycoplasma genitalium infections due to macrolide and fluoroquinolone resistance. 2015; doi.org/10.1177/0956462413502008.
- Jensen JS., Bradshaw CS., Tabrizi SN, Fairley CK, Hamasuna R. Azithromycin treatment failure in Mycoplasma genitalium-positive patients with nongonococcal urethritis is associated with induced macrolide resistance. Clin Infect Dis. 2008;47(12):1546–1553.
- Twin J, Jensen JS, Bradshaw CS, et al. Transmission and selection of macrolide resistant Mycoplasma genitalium infections detected by rapid high resolution melt analysis. PLoS ONE. 2012;7(4): e35593. doi:10.1371/journal.pone.0035593.
- Chen X, Sullivan PF. Single nucleotide polymorphism genotyping: biochemistry, protocol, cost and throughput. The Pharmacogenomics Journal. 2003;3(2):77–96.
- Tan LY, Walker SM Lonergan T, et al. Superior multiplexing capacity of plexprimers enables sensitive and specific detection of SNPs and clustered mutations in qPCR. Plos One. 2017;12(1):e0170087.
- Tabrizi SN, Tan LY, Walker S Twin, et al. Multiplex assay for simultaneous detection of Mycoplasma genitalium and macrolide resistance using Plexzyme and Plexprime technology. Plos One. 2016;11(6):he0156740.
- Lau A, Bradshaw CS, Lewis D, et al. The efficacy of azithromycin for the treatment of genital Mycoplasma genitalium : a systematic review and meta-analysis. Clin Infect Dis. 2015;61(9):1389–1399.
- Manhart LE. Diagnostic and resistance testing for Mycoplasma genitalium: what will it take? Clin Infect Dis. 2014;59(1):31–33.
- Nijhuis, RHT, Severs TT, Van der Vegt, DS. J. M., Van Zwet AA, Kusters JG. High levels of macrolide resistance-associated mutations in Mycoplasma genitalium warrant antibiotic susceptibility-guided treatment. J Antimicrob Chemother. 2015;70(9):2515-2518.
- Manhart LE, Critchlow CW, Holmes KK, Dutro SM, et al. Mucopurulent cervicitis and Mycoplasma genitalium. J Infect Dis. 2003;187(4):650–657.
- Oakeshott P, Aghaizu A, Hay P, et al. Is Mycoplasma genitalium in women the “new chlamydia”? a community‐based prospective cohort study. Clin Infect Dis. 2010;51(10): 1160–1166.
- Sonnenberg P, Ison CA, Clifton S, et al. Epidemiology of Mycoplasma genitalium in British men and women aged 16–44 years: evidence from the third National Survey of Sexual Attitudes and Lifestyles (Natsal-3). International Journal of Epidemiology. 2015; dyv194.
- Hardick J, Giles J, Hardick A, et al. Performance of the gen-probe transmission-mediated amplification research assay compared to that of a multitarget real-time PCR for Mycoplasma genitalium detection. J Clin Microbiol. 2006;44:1236–1240.
- Gaydos C, Maldeis NE, Hardick A, Hardick J Quinn TC. Mycoplasma genitalium as a contributor to the multiple etiologies of cervicitis in women attending sexually transmitted disease clinic . Sex Transm Dis. 2009; 36(10):598–606.
- Wetmore CM, Manhart LE, Lowens MS, et al. Demographic, behavioral, and clinical characteristics of men with nongonococcal urethritis differ by etiology: a case-
comparison study. Sex Transm Dis. 2011;38(3):180-186. - Yew HS, Anderson T, Coughlan E, Werno A. Induced macrolide resistance in Mycoplasma genitalium isolates from patients with recurrent nongonococcal urethritis. J Clin Microbiol. 2011; 49(4):1695–1696.
- Manhart LE, Holmes KK, Hughes JP, Houston LS, Totten PA. Mycoplasma genitalium among young adults in the United States: an emerging sexually transmitted infection. Am J Public Health. 2007;97(6):1118-1125.
- Walker J, Fairley CK, Bradshaw CS, et al. The difference in determinants of Chlamydia trachomatis and Mycoplasma genitalium in a sample of young Australian women. BMC Infect Dis. 2011 Feb 1;11:35. http://bmcinfectdis.biomedcentral.com/articles/10.1186/1471-2334-11-35.
- O’Neill J. Tackling drug-resistant infections globally: final report and recommendations. The Review on Antimicrobial Resistance. May 2016:35.
Litty Tan, BSc, PhD, has more than 10 years’ experience in medical research, and she has extensive expertise in molecular assay development for cancer and infectious disease diagnostics. She serves as the Director of Research and Development at SpeeDx Pty. Ltd. In this role she manages research projects and coordinates product development and product evaluations to ensure quality and regulatory compliance.