Whether your laboratory conducts proficiency testing (PT) as required by the Centers for Medicare & Medicaid Services CLIA regulations (42 CFR 483 for Subpart H) for non-waived tests, or as part of verification for tests and/or as part of process improvement, proficiency testing programs evolve over time. Such programs can be affected by new technologies and methodologies; diagnostic testing options for personalized medicine; availability of laboratory developed tests (LDTs); changes in the clinical laboratory workforce; increased automation and LIS interoperability; and growth of genetic testing laboratories.
For laboratories conducting testing that requires participation in a PT survey program to comply with CLIA, 42 CFR 493 Subpart H – Participation in Proficiency Testing for Laboratories Performing Nonwaived Tests, Subpart I identifies the specialties, subspecialties, and specific analytes or tests that require testing participation. CLIA regulations apply to FDA cleared and approved assays as well non-FDA cleared and approved assays. Under CLIA, genetic tests (cytogenetics) are specifically listed, but should be included in the specialty or subspecialty where they fit best, according to the methodology of the test.
Technologies
The rapid development of new technologies and methodologies poses a challenge for both laboratories conducting PT testing and PT program survey sample providers. This is particularly true for recent advances in molecular, genomic, multiplex, and/or microarray technology. This is also true for the rapid identification of new disease associations and predictors; and new alleles, variants or mutations associated with disease (for example, risk, susceptibility, treatment effect), which have allowed for more personalized medicine.
Personalized medicine
The development of personalized medicine allows patient medical assessment and treatment to be individually tailored for a given patient and his or her disease. Personalized medicine can include the use of predictive tests (for example, genetic testing for predictors of disease or treatment effect; companion diagnostics to a therapeutic treatment). Companion diagnostics devices1 can identify patients who are most likely to benefit from a therapeutic product or who may be at increased risk for treatment side effects, and/or can be used to monitor a treatment effect. Companion diagnostics are genomic-based tests, and they may be FDA cleared or approved, or LDTs.
Genetic testing
The CLIA regulations do not specifically cover genetic testing laboratories, and therefore for this type of laboratory there is no requirement for PT. However, this is where PT testing as part of verification for regulated and non-regulated tests and/or as part of process improvement applies and should be considered.
There are thousands of molecular genomic tests for individual disorders. Next generation DNA sequencing and other novel technologies (for example, microarrays, mutation scanning) may include complex and novel chemical, hardware, and/or software elements. New analysis tools are often required to interpret the results of such testing. This takes the complexity of adequate PT testing to a new level.
Point-of-care testing
Proficiency testing for point-of-care (POC) diagnostics may pose another challenge. Many POC tests are waived and therefore do not fall under the CLIA regulations. However, some non-waived tests may be performed in a satellite laboratory or patient care area, and are therefore considered POC testing. Such testing sites have accreditation and regulatory implications, as the location of the testing is not relevant. It is important to ensure that these locations are included in a PT program.
Clinical laboratory workforce changes
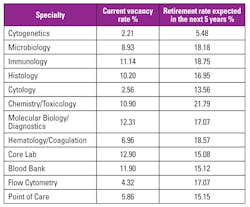
There has been concern about the aging of the clinical laboratory workforce and the retirement of experienced laboratorians. In 2011, an estimate of medical laboratory professional vacancy rates by 2018 was cited as potentially being as high as 40 percent.2 The American Society for Clinical Pathologists 2014 Vacancy Survey of Clinical Laboratories in the United States3 collected data on current vacancy rates and expected retirement rates over the next five years (Table 1). While not 40 percent, it may be expected that the large retirement rates may result in increasing vacancy rates.
In 2015, the job growth potential for medical and clinical laboratory technologists and technicians was estimated to be 16 percent between 2014 and 2024.4 This growth may be a result of a combination of elements, including the retiring clinical laboratory population (resulting in position availability) and an increase in diagnostic procedures for an aging general population and individuals with health insurance (resulting in an increase in laboratory testing).
What is the impact of a change in the level of workforce experience on PT? Historically, proficiency in testing was known to be negatively affected by lack of experience. But that is not the complete story today.
Automation
Laboratory automation has both a potential positive and potential negative impact on proficiency testing.
As for the positive aspect, the increase in automation may mitigate some of the effect of PT failure associated with inexperienced staff or testing that is not done routinely. In this case, stringent specialized qualifications or training may not be required, and the testing technique, result interpretation, and data analysis may be relegated to the instrument/testing platform and/or software solutions. This is likely to decrease the risk of failed PT.
As for the negative, the number of the same manufacturer’s instrument a laboratory may have, where each performs the same test(s), and/or the number of instruments from different manufacturers that perform the same test(s), may result in complexity in the scheduling of PT. This is due to the need to ensure inclusion of all instruments (from the same or different manufacturers); testing of the same analyte on different instrument types; testing of all analytes (which may require multiple survey panels); and testing of all staff.
Challenges of survey sample availability
Some of these new technologies or methodologies may be conducted by a limited number of laboratories, may include laboratory developed tests, and/or may require specialized samples (for example, samples for rare analyte targets). In these cases, a national, formal PT survey may be impractical, if not impossible, and proficiency assessment becomes the responsibility of the laboratory. Solutions may include use of samples from non-accredited programs, use of well-characterized internal samples or samples from collaborative institutions, and/or use of well-characterized samples from the assay manufacturer.
The ever-changing landscape of laboratory testing and the ability to successfully perform PT is a challenge that laboratorians have successfully met for decades, and will continue to meet.
REFERENCES
- U.S. Food ND Drug Administration. Companion Diagnostics located at: http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/InVitroDiagnostics/ucm407297.htm.
- Lacey-Mabe C. Medical laboratory professionals workforce report. Advance Healthcare Network Jobs web page. http://www.advanceweb.com/jobs/news-by-profession/laboratory-science-articles/medical-laboratory-professionals-workforce-report.html.
- Garcia E, Ali AM, Soles RM, The American Society for Clinical Pathology’s 2014 Vacancy Survey of Medical Laboratories in the United States. AJCP 2015, 144:432-443.
- U.S Department of Labor, Bureau of Labor Statistics. Medical and Clinical Laboratory Technologists and Technicians. Occupational Outlook Handbook. https://www.bls.gov/ooh/healthcare/medical-and-clinical-laboratory-technologists-and-technicians.htm.
Sharyn Orton, MT(ASCP)SBB, MSPH, PhD, serves as a Senior Consultant for Massachusetts-based MEDIcept, Inc.