The continuing case for point-of-care testing for HbA1c
There is an ongoing conflict between traditional clinical laboratories and the relative new kid on the block, point-of-care testing (POCT). Of course, the laboratory system will likely always be king. But there is absolutely a place for POCT, especially as the way in which we approach healthcare, especially diagnostics, develops beyond the usual settings.
POCT ensures the rapid provision of diagnostic information, ideally during one consultation, to enable clinical decisions to be made at the earliest opportunity. Such rapid provision of information facilitates optimization of the care process. The potential for any application of POCT can, therefore, be judged in terms of its contribution to decision making and to the process of care.
In the case of the management of diabetes patients, POCT for glycated hemoglobin (HbA1c) may offer a number of advantages—as long as the performance characteristics of the analyzers used are equivalent to those employed in the central laboratory, and can be certified as such.
The use of HbA1c for management of diabetes
Glycated hemoglobin (HbA1c) is well-recognized as a reliable measure for glycemic control. The role of HbA1c testing in the management of patients with diabetes has been established for several decades, while its role in the diagnosis of diabetes has been documented more recently.
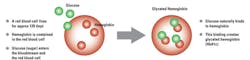
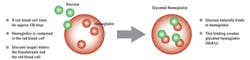
These applications are based on the fact that HbA1c levels reflect the average circulating glucose concentration over the lifespan of red blood cells (two to three months). This is because the glycated hemoglobin molecule in blood is highly stable once it has formed after exposure to glucose, since glucose binds irreversibly in a non-enzymatic reaction to the N-terminal valine of hemoglobin A in red blood cells (Figure 1). Therefore, use of HbA1c can offer greater clinical information than a single glucose measurement taken at a particular time. Furthermore, there is evidence that HbA1c is a good predictor of an individual’s risk of developing long-term complications of diabetes, e.g., cardiovascular disease.1-3
Serial measurements of HbA1c can show how an individual’s glucose control (and hence risk of complications) change in response to alterations in management over time. It is recommended that HbA1c should be measured every two to six months, with target HbA1c levels set for individual patients and therapy adjusted accordingly.4 General targets of HbA1c for diabetic individuals, depending on their risk of severe hypoglycemia, cardiovascular status, and co-morbidities, should be set between 6.5 percent and 7.5 percent (48-58 mmol/mol), with the non-diabetic reference range being 4.0 percent to 6.0 percent (20-42 mmol/mol). Within the United Kingdom, for example, current National Institute for Health and Clinical Excellence (NICE) guidelines stipulate that, providing there is no disabling hypoglycemia, the target HbA1c concentration for children, young people, and adults with type 1 diabetes is 7.5 percent, and that additional support should be offered if HbA1c is consistently greater than 9.5 percent.
One consideration is that HbA1c results may be affected by any condition that leads to a change in red blood cell survival; however, even then, HbA1c can be used to detect trends in a patient’s glycemic control rather than for target setting.
The growing case for HbA1c in diabetes diagnosis
The attributes of HbA1c measurement for the management of diabetes are equally applicable for use in the diagnosis of diabetes. Furthermore, the performance of HbA1c has been shown to be equal to that of fasting blood glucose tests commonly used for type 2 diabetes screening.5
Unlike glucose levels, which are affected by what a patient has eaten and drunk in the previous two to three hours, HbA1c levels do not require a patient to fast prior to the test. Consequently, as a simple and immediate test for diabetes, POC HbA1c can support early identification of at-risk individuals. This would then rapidly enable them to make changes to their lifestyle, in order to significantly reduce the risk of developing type 2 diabetes or eliminate it altogether. The ability to rapidly assess and change risk outcomes has significant health benefits for the patient and also reduces overall healthcare costs related to the complications of type 2 diabetes. It is known that patients diagnosed with diabetes who maintain low blood HbA1c levels significantly reduce the onset of complications after diagnosis.6
The World Health Organization has recommended the use of HbA1c for the diagnosis of diabetes,7 and similar guidance has followed in several countries.8,9 In the UK, for example, NICE published guidelines in 2013 for diabetes prevention which aim to identify people at high risk of type 2 diabetes and to offer cost-effective and appropriate interventions to prevent or delay onset.8 Used in conjunction with a lifestyle health risk assessment, these guidelines advocate the use of HbA1c levels to allow healthcare providers to advise individuals on treatment regimens, depending on their classification as low, moderate, or high risk of developing type 2 diabetes. NICE has since followed up with new guidelines in 2015 for the management of type 2 diabetes in adults which suggests HbA1c levels should be tested at three-to-six month intervals and six-month intervals once a stable HbA1c level has been obtained.9
Likewise in the United States, a recent American Diabetes Association workgroup report concluded that the HbA1c assay is an accurate, precise measure of long-term glycemic levels that correlates well with diabetes complications and offers several advantages over laboratory measures of glucose.10 However, it should be noted that use of HbA1c for diabetes diagnosis is not appropriate for all individuals. The test should not be used in children, young people, pregnant women, individuals in whom type 1 diabetes is suspected, individuals whose symptoms have been of short duration, or patients who are acutely ill.11,12
It has also been suggested that the cut-off value for diabetes diagnosis generally quoted, 6.5 percent (48 mmol/mol), may not be appropriate for all populations. Additionally, individuals within the range of 6.0 percent to 6.4 percent (42-47 mmol/mol) should be considered at high risk of developing diabetes and be advised appropriately and retested annually. Those with values less than 6 percent (42 mmol/mol) should be tested every three years.12
Current guidance, therefore, supports the employment of HbA1c measurement in both screening for type 2 diabetes and in the management of patients with diabetes. Use of POCT could improve management of patients with established diabetes in both primary and secondary care settings and also enable earlier type 2 diabetes diagnosis.
HbA1c in POCT-based diabetes monitoring
Early HbA1c determination was based on laboratory-based methods including ion exchange and affinity chromatographic methods, with alternative affinity and immunological methods following later, taking HbA1c into the point-of-care environment.
There is certainly a strong case for employing POC-based HbA1c testing for diabetes monitoring, since lying at the heart of the concept of POCT is the principle that medical tests are convenient and immediate to the patient. Typically, patients with existing diabetes are monitored for HbA1c every three to six months. This generally involves a nurse or phlebotomist visit for venepuncture, with a follow-up appointment one to two weeks later to discuss results once they are available from the laboratory.
From a patient experience perspective, use of POCT for HbA1c can enhance satisfaction levels. This is because use of POCT means that after one visit they can leave with an immediate action plan or relevant prescription, should results indicate the need. Furthermore, enabling earlier therapeutic decisions may result in improved diabetic control, better patient outcomes, and enhanced clinic efficiency, with fewer patient visits and subsequent economic benefits. For example, a Swedish before-and-after study investigated the economic costs and benefits of implementing HbA1c home testing.13,14 It confirmed a reduction in costs due to fewer clinic visits, reduction in total treatment costs, time saved resulting from reduced labor costs in both administration and sampling, and reduced travel costs—as well as a reduction in mean HbA1c levels.
In addition, four observational studies of more than 5,700 patients with diabetes, in which there was immediate feedback of results to patients, also showed significant reductions in HbA1c levels.15-18 One of these studies demonstrated maintenance of improved HbA1c levels for a period of four years.17 A recent systematic review of quality improvement (QI) strategies in the management of diabetes demonstrated that QIs involving greater adherence to guidelines can help improve HbA1c levels.8 There is also good evidence to show that patient satisfaction is improved using POCT and that immediate knowledge of a patient’s HbA1c levels is associated with better outcomes, as judged by reduced HbA1c levels.16-24
What to look for in a POC HbA1c analyzer
Most POC HbA1c analyzers use a drop of blood (4 to10 µL) taken from a finger prick (capillary blood) or venous sample that is applied to a reagent cartridge and then inserted into a desktop device for analysis. Time to reporting of HbA1c results is generally between three and 10 minutes, depending on the analyzer. Keys to the effectiveness of any HbA1c POC analyzer are simplicity, audit trails, certification, and methodology:
Simplicity. Ensuring that a POC analyzer is as easy to use as possible will minimize the chance of user error and hence the need for retesting, with subsequent time and cost implications. An analyzer that is simple to use also ensures minimal training requirements, again contributing to affordability.
Features that can support ease of use include the employment of ready-to-use reagent cartridges that can be inserted straight into the analyzer with the blood sample then added directly, without the need for sample preparation such as premixing or pipetting. Minimizing the number of steps in the procedure not only reduces the opportunity for user error but also helps to standardize results by eliminating any variation introduced by different operators, particularly when pipetting and mixing.
Audit trails. For patient safety purposes, audit trails should also be available. Barcode scanning for patient and user identification, as well as for confirmation of the batch of reagents and controls used, ensures that an analyzer can provide such trails. Quality control results on two levels that are recorded and held within the analyzer’s memory are also ideal for auditing purposes.
Certification. Certification of the analyzer in order to confirm delivery of accurate, standardized results should also be a key consideration. In an effort to standardize HbA1c results, the American Association of Clinical Chemistry (AACC) set up the National Glycohemoglobin Standardization Program (NGSP) in 1996. In parallel, the International Federation of Clinical Chemistry (IFCC) developed reference methods for glycated hemoglobin. In 2006 and 2007, an international consensus between IFCC and AACC was reached.25
The calibration and certification of laboratories and manufacturers to the same standards has improved the conformity of results. However, in practice differences can still be observed among technologies and among individual systems. These observed differences arise because of heterogeneity of hemoglobins, underlying differences in technologies (e.g., ion exchange, boronate affinity, immunoassay), calibration drifts, or lot-to-lot variability. However, if the manufacturer follows the recommendations of the IFCC and NGSP to ensure instruments and reagents are accurately aligned and traceable to the reference method, this should not prove problematic.
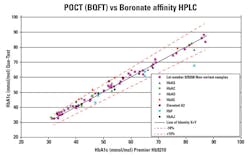
Methodology. There are POC HbA1c analyzers available whose results are not affected by hemoglobin variants (which do not result in reduced erythrocyte life span), labile glycated hemoglobin, or hematocrit levels. Such analyzers use boronate fluorescence quenching technology (BFQT),26 which is associated with multiple optical measurements. This methodology is traceable to well-documented boronate affinity HPLC systems used in reference laboratories. However, as BFQT does not require chromatographic separation, the methodology allows for accurate POC measurement of HbA1c to deliver results comparable to chromatography-based techniques. Recent evidence suggests that the latest POCT systems deal with hemoglobin variants equally as well as HPLC systems.27 (Figure 2).
Conclusion
There are strong arguments for the use of POCT for HbA1c where the performance characteristics of the systems are equivalent to those employed in the central laboratory (or even better in some cases) and are certified as such. POCT offers improved access to testing, as well as enabling immediate clinical decision making, discussion with the patient, and implementation of appropriate treatment and/or lifestyle advice. Further, as POCT enables testing to be undertaken closer to patients, it affords greater convenience for them, thereby improving the likelihood of treatment compliance by empowering them. This should
ensure better glycemic control, which is the ultimate goal.
REFERENCES
- Khaw KT, Wareham N, Bingham S, Luben R, Welch A, Day N. Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Ann Intern Med. 2004;141:413–420.
- Selvin E, Marinopoulos S. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann of Internal Medicine. 2004;141:421–431.
- ten Brinke R, Dekker N, de Groot M, Ikkersheim D. Lowering HbA1c in type 2 diabetics results in reduced risk of coronary heart disease and all cause mortality. Prim Care Diabetes. 2008;2:45–49.
- Diabetes UK. HbA1c Standardisation: Information for Clinical Healthcare Professionals. 2009. http://www.diabetes.org.uk/Guide-to-diabetes/Monitoring/Blood_glucose/Glycated_haemoglobin_HbA1c_and_fructosamine/HbA1c_Standardisation_Information_for_Clinical_Healthcare_Professionals. Accessed May 21, 2013.
- Bennett CM, Guo M, Dharmage SC. HbA(1c) as a screening tool for detection of Type 2 diabetes: a systematic review. Diabet Med. 2007;24:333-343.
- Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-986.
- World Health Organization. Use of glycated haemoglobin (HbA1c) in the diagnosis of diabetes mellitus. 2011. www.who.int/diabetes/publications/report-hba1c_2011.pdf. Accessed May 21, 2013.
- National Institute for Health and Clinical Excellence. Preventing type 2 diabetes: risk identification and interventions for individuals at high risk. 2012. www.nice.org.uk/nicemedia/live/13791/59951/59951.pdf. Accessed May 21, 2013.
- NICE Guidelines: Type 2 diabetes in adults: management. 2015. https://www.nice.org.uk/guidance/ng28.
- American Diabetes Association. International Expert Committee Report on the role of the A1C Assay in the diagnosis of diabetes. Diabetes Care. 2009;32(7):1327-1334.
- Inzucchi SE. Clinical practice. Diagnosis of diabetes. N Engl J Med. 2012;367:542-550.
- Farmer A. Use of HbA1c in the diagnosis of diabetes. BMJ. 2012;345:e7293.
- Snellman K, Eckerborn S. Possibilities and advantages of HbA1c: eight years experience. Diabet Med. 1997;14(5):401-403.
- Plüddemann A, Price CP, Thomson M, Wolsteholme J, Heneghan C. British Journal of General Practice. 2011;61:139-140.
- Grieve R, Beech R, Vincent J, Mazurkiewcz J. Near patient testing in diabetes clinics: appraising the cots and outcomes. Health Technol Assess. 1999;3:1-74.
- Ferenczi A, Reddy K, Lorber DL. Effect of immediate hemoglobin A1c results on treatment decisions in office practice. Endocr Pract. 2001;7:85–88.
- Petersen JR, Finley JB, Okorodudu AO, Mohammad AA, Grady JJ, Bajaj M. Effect of point-of-care on maintenance of glycemic control as measured by A1C. Diabetes Care. 2007;30:713–715.
- Rust G, Gailor M, Daniels E, McMillan-Persaud B, Strothers H, Mayberry R. Point of care testing to improve glycemic control. Int J Health Care Qual Assur. 2008;21:325–335.
- Driskell OJ, Holland D, Hanna FW, et al. Inappropriate requesting of glycated hemoglobin (Hb A1c) is widespread: assessment of prevalence, impact of national guidance, and practice-to-practice variability. Clin Chem. 2012;58:906-915.
- Peterson KA, Radosevich DM, O’Connor PJ, et al. Improving Diabetes Care in Practice: findings from the TRANSLATE trial. Diabetes Care. 2008;31:2238-2243.
- Laurence CO, Gialamas A, Bubner T, et al; Point of Care Testing in General Practice Trial Management Group. Patient satisfaction with point-of-care testing in general practice. Br J Gen Pract. 2010;60:e98–104.
- Cagilero E, Levina EV, Nathan DM. Immediate feedback of HbA1c levels improves glycemic control in type 1 and insulin-treated type 2 diabetic patients. Diabetes Care. 1999;22(11):1785-1789.
- Miller CD, Barnes CS, Phillips LS, et al. Rapid A1c availability improves clinical decision-making in an urban primary care clinic. Diabetes Care. 2003;26(4):1158-1163.
- Kennedy L, Herman WH, Strange P, et al; GOAL A1C Team. Impact of active versus usual algorithmic titration of basal insulin and point-of-care versus laboratory measurement of HbA1c on glycemic control in patients with type 2 diabetes: the Glycemic Optimization with Algorithms and Labs at Point of Care (GOAL A1C) trial. Diabetes Care. 2006;29(1):1-8.
- Geistanger A, Arends S, Berding C, et al; on behalf of the IFCC Working Group on Standardization of Hemoglobin A1c. Statistical Methods for Monitoring the Relationship between the IFCC Reference Measurement Procedure for Hemoglobin A1c and the Designated Comparison Methods in the United States, Japan, and Sweden. Clin Chem. 2008;54(8):1379-1385.
- Wilson DH, Bogacz JP, Forsythe CM, et al. Fully automated assay of glycohemoglobin with the Abbott IMx analyzer: novel approaches for separation and detection. Clin Chem. 1993;39(10):2090-2097.
- Lenters-Westra E. An evaluation of the Quo-Test® performance against NGSP criteria and sigma-metric. 2016.
Gavin Jones, BSc(Hons) in Forensic and Biomolecular Sciences, serves as Product Manager (Diabetes) at EKF Diagnostics, which specializes in the development, production, and distribution of point-of-care analyzers for use in the detection and management of diabetes, anemia, lactate, and kidney-related diseases.