An update on Vitamin D testing: What is right for your laboratory?
Interest in vitamin D testing has grown exponentially during recent years. Scholarly publications have linked vitamin D to a range of issues related to cardiology, cancer, pregnancy, metabolic disorders, mortality, and more. Vitamin D testing creates opportunities, but also challenges, for the clinical laboratory.1
25-OH Vitamin D is a challenging analyte to accurately measure in human blood due to the presence of 25-OH Vitamin D2, Vitamin D binding proteins, 25-OH Vitamin D metabolites such as 24, 25 (OH)2 Vitamin D, and C-3 epimers of 25-OH Vitamin D.1 Variability among methods due to lack of standardization has added to the challenges.
To address this need, in 2010 the National Institute of Health’s Office of Dietary Supplements (NIH ODS) introduced the Vitamin D Standardization Program (VDSP), an initiative to standardize the laboratory measurement of vitamin D status worldwide.3, 4
While standardization harmonizes the test results by reducing the variability among test results from different manufacturers, it does not address the variability due to antibody differences.
In an article published in this space in October 2013, “Vitamin D testing: What is right for your laboratory” (MLO. 2013:45(10):32-34), I discussed the several factors that could cause discrepancy in vitamin D results and how laboratorians need to look carefully to determine whether a given assay is a good fit for their laboratory and patient population. In this article, I will discuss the new generation of vitamin D immunoassays that are standardized to the NIST SRM 2972.
Standardization of Vitamin D assays
To overcome the problem of variability between different 25-OH Vitamin D methods, the VDSP was established in 2010. VDSP is in collaboration with National Institute of Standards and Technology (NIST), the Centers for Disease Control and Prevention (CDC), and Ghent University in Belgium. The NIST and Ghent reference measurement procedures are the reference methods for the measurement of Total 25-OH Vitamin D; i.e., 25-OH Vitamin D2 and D3. The NIST, in collaboration with the ODS, has developed and certificated Standard Reference Materials (SRM 2972 and 972a) for vitamin D metabolites in human serum.3, 4
The recent release of a new generation of standardized 25-OH Vitamin D immunoassays, which are aligned to the NIST SRM 2972, leaves a perception that these assays show improved analytical performance. Considering the difficulties of 25-OH Vitamin D measurements in the past, the assumption was that these standardized immunoassays will represent an improvement in inter-laboratory as well as inter-assay discrepancy.
Equimolar detection of 25-OH Vitamin D3 and D2
The anticipation was that with the standardization of 25-OH Vitamin D assays, the issue of non-equimolar detection of 25-OH Vitamin D3 and D2 would be resolved and immunoassays would be aligned to LC-MS/MS. But in fact, this continues to be an issue after the standardization.2, 5-8
The factor to be considered is the approach used by manufacturers to the standardization of assays. Many manufacturers have harmonized standardization to make their results traceable to the reference measurement procedure (RMP). Several manufacturers have re-calibrated their assays and have obtained certification by the CDC Standardization-Certification Program. However, differences continue to exist among assays, and there has been considerable variability of bias among samples by the same method.
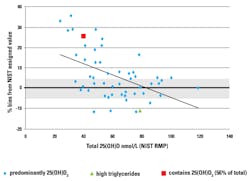
Although Vitamin D External Quality Assessment Scheme (DEQAS) results have shown improved agreement among participating laboratories, there is still considerable variability among commercial methods.2, 5-8 A recent review of Vitamin D DEQAS results showed that methods do not show equimolar detection of 25-OH Vitamin D3 and D2 in spite of standardization.2
The DEQAS also showed that bias of results may be concentration-dependent, and four out of five immunoassays compared had a positive association between bias and 25-OH Vitamin D concentration2 (Table 1, Figure 1, Figure 2).
Cross reactivity to 24, 25 (OH)2 Vitamin D.2,6
In patients with high levels of Vitamin D, the 25-OH Vitamin D is hydroxylated to 24, 25, (OH)2 Vitamin D, which can be regarded as a physiological mechanism to prevent hypercalcemia. The potential interference of 24, 25 (OH)2 Vitamin D is of interest to clinical laboratories.
The presence of dihydroxylated Vitamin D metabolites [eg. 24, 25(OH)2 Vitamin D] may falsely elevate 25(OH) Vitamin D results, particularly at high concentrations (Table 2).
25-OH Vitamin D published guidelines
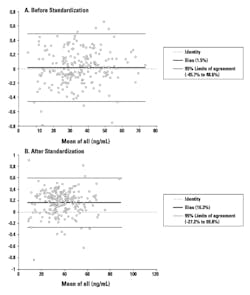
Published guidelines for the interpretation of 25-OH Vitamin D results are not method-specific and assume harmonization among the different methods. However, it is clear that there still exists considerable bias between methods even though the re-standardized assays are now traceable to the NIST SRM 2972.2, 5-8
The within-method variability is possibly due to matrix effects and differences in Vitamin D binding protein (DBP). The between-method variability is possibly due to antibody specificity and assay design.
Even after standardization and harmonization, the differences in performance remain a concern. Clinicians should be aware when comparing results among assays and using them to determine 25-OH Vitamin D status in various patient populations. NIST-SRM aligned methods do not necessarily imply that results are accurate. Careful consideration of assay design and interferences is necessary, along with other pre-analytical factors affecting 25-OH Vitamin D results.
REFERENCES
- Herrmann M. The measurement of 25-hydroxy vitamin D–an analytical challenge. Clin Chem Lab Med. 2012;50(11):1873-1875.
- Carter G, Jones J, Walker E; Assays for vitamin D metabolites; performance in the real world. European Vitamin D Association Conference papers.
- Sempos, Christoper; Standardization of vitamin D Assays: The past and present dilemma for vitamin D research. European Vitamin D Association Conference papers.
- Thienpont LM, Stepman H, Vesper HW. Standardization of measurements of 25-hydroxyvitamin D3 and D2. Scand J Clin Lab Invest. 2012;72(Suppl 243):41-49.
- Enko D, Kriegshauser G, Stolba R, Worf E, Halwachs-Baumann G; Method evaluation study of a new generation of vitamin D assays; Biochemia Medica. 2015;25(2):203-212.
- Discrepancy between Vitamin D Total Immunoassays due to Various Cross-reactivities.
- Schottker B, Jansen E, Haug U, Schomburg L, Kohrle J, Brenner H; Standardization of misleading immunoassay based 25-Hydroxyvitamin D levels with liquid chromatography tandem-mass spectrometry in a large cohort sStudy; PLOS ONE November 2012 | Volume 7 | Issue 11 | e48774.
- Wyness S, Straseski J; Performance characteristics of six automated 25-hydroxyvitamin D assays: Mind your 3s and 2s; Clin Biochem. 2015;48(16-17):1089-1096.
Shanti Narayanan, MS, MBA, serves as marketing manager for California-based Tosoh Bioscience, Inc. She can be reached at [email protected].