Diabetes is a worldwide epidemic. Its prevalence continues to rise globally at an average rate of 8.7 percent, and it currently affects 382 million of the world’s population. Significant increases in populations diagnosed with diabetes have been reported by many nations as their lifestyle and dietary norms evolve with globalization. National healthcare budgets bear the financial burden of treating diabetes and its complications, exceeding $548 billion dollars globally.1
Through the power of diagnostic testing to help screen, diagnose, and monitor, a patient’s chronic condition can be kept in balance and not allowed to escalate to a critical state that lessens quality of life and may require hospitalization and more expensive intervention.
The impact of diabetes
Diabetes is defined as a chronic disease that occurs when the pancreas is no longer able to make insulin or when the body cannot make good use of the insulin it produces. Not being able to produce insulin or use it effectively leads to raised glucose levels in the blood (known as hyperglycemia). Over the long-term, high glucose levels are a threat to well-being, and are associated with damage to the body and failure of various organs and tissues.
Alarmingly, the growing number of people with diabetes worldwide will place even more individuals at risk for developing the comorbidities associated with diabetes. Left untreated, complications may affect the proper functioning of all organ systems, including increases in the likelihood of cardio-renal syndrome exhibited by progressive chronic kidney disease (CKD) and cardiovascular disease (CVD) that result in premature mortality.2
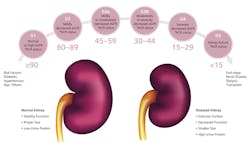
Diabetes is the leading cause of CKD,2 which is more prevalent in diabetics than non-diabetics.3 Uncontrolled high blood glucose and high blood pressure cause damage to small blood vessels, leading to decreased kidney function.4
Kidney failure is the most costly chronic disease, accounting for five percent of annual healthcare budgets (over $30 billion in the U.S. alone). People with early CKD are generally asymptomatic; hence, early detection and treatment are crucial for preventing or slowing progression to end stage renal disease (ESRD), complications, and premature death.5
The power of HbA1c and ACR
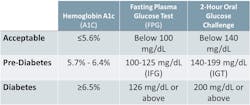
Hemoglobin A1c (HbA1c) is recognized as a reliable and convenient biomarker for the screening, diagnosis, and management of long-term diabetes. It is recommended as a marker for the management of diabetes in CKD patients2,6; the prevalence of diabetes-related complications rises at higher HbA1c (> ~six percent to seven percent). Glycemic control, as reflected by normoglycemic HbA1c concentrations, leads to reduction in diabetic complications, including nephropathy.4
Higher levels of urine albumin (albuminuria) are determined by a measurement of urine albumin to creatinine ratio (ACR). They are possibly the earliest indication of diabetic and other glomerular kidney diseases and are associated with all-cause and CV mortality,7 adverse outcomes (ESRD, acute kidney injury, and progression of CKD), and mortality in CKD patients.8
CKD is defined as abnormalities in kidney structure or function of greater than three months. The Kidney Disease Improving Global Outcomes (KDIGO) guidelines recommend classifying CKD as to (1) Cause, (2) GFR category, and (3) Albuminuria category (CGA). A CKD diagnosis is made when one or both of the following are present for greater than three months: 2
Additionally, guidelines recommend that all CKD patients, including children, should be considered at increased risk for CVD because lower eGFR and abnormally high levels of albumin in the urine associated with CKD are also associated with cardiovascular mortality.2,7,9 The most common cause of death in the dialysis population is CVD; CVD mortality is twice as high in dialysis patients as in the general population.5 Increased risk for CVD is observed in the early stages of CKD.10, 11
Monitoring for comorbidities
Diabetes is a multi-faceted chronic illness requiring continuous monitoring with multi-faceted risk reduction strategies. Today, physicians require immediate access to the diagnostic tools that provide comprehensive data to diagnose and evaluate the progression of diabetes, CKD, and CVD.
Physicians require the sensitivity and specificity of specialty assays performed in the clinical laboratory. Tests such as HbA1c, c-peptide, insulin, glycated albumin, and glucose each have their role as laboratory tests, including diagnosis and monitoring for diabetes. Creatinine, eGFR, cystatin c, and urine albumin are key assays to assess the progression of CKD within the KDIGO guidelines.
Markers to aid in the assessment of cardiovascular events and disease include BNP (B-type natriuretic peptide), NT-proBNP (N-terminal pro-brain natriuretic peptide), and cardiac troponin (cTn).2
POC enhances management
Physicians’ ability to perform key assays such as HbA1c and albumin/creatinine ratio in-office is important to establish an effective consultative relationship with patients and to be able to consider timely therapy modifications to enhance well-being and quality of life.
As healthcare becomes more connected, so has the in vitro diagnostic industry. The harmonization and standardization of cost-effective end-to-end solutions across reference labs, hospitals, clinics, and physician offices ensure consistent, accurate, and reliable results regardless of where testing takes place.
Point-of-care (POC) informatics can maintain control and visibility from a central location to facilitate high-quality results and enhanced patient outcomes. Although there remains a resource disparity in many regions of the world that can affect the level of care, the availability of innovative technologies surrounding informatics is empowering physicians and patients with ready delivery of customizable reports, remote 24/7 monitoring, and control support services.
The future is here. Diagnosis, screening, and monitoring the progression and treatment of diabetes has been shaped and transformed by the delivery of clinical and workflow excellence through end-to-end solutions including informatics, automation, and pre-post analytics. These innovations help enable patient treatment that can advance human health today.
References
- International Diabetes Federation, IDF Diabetes Atlas, Sixth Edition, 2013:31.
- Levin A, Stevens PE, Co-chairs: Kidney Disease: Improving Global Outcomes Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney International Supplements 2013;3:v-150.
- Plantinga LC, Crews DC, Coresh J, et al. Prevalence of chronic kidney disease in US adults with undiagnosed diabetes or prediabetes. Clin J Am Soc Nephrol. 2010;5:673-682.
- Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008; 358:2560-2572.
- United States Renal Data System, 2014 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. National Institutes of Health, National Institute of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2014.
- Standards of Medical Care in Diabetes—2015. Diabetes Care. 2015;38:S1-92.
- van der Velde M, Matsushita K, Coresh J, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int. 2011;79:1341-1352.
- Astor BC, Matsushita K, Gansevoort RT, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int. 2011;79:1331-40.
- Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073-2081.
- Culleton BF, Larson MG, Wilson PW, Evans JC, Parfrey PS, Levy D. Cardiovascular disease and mortality in a community-based cohort with mild renal insufficiency. Kidney Int. 1999;56:2214-2219.
- Zhang L, Zuo L, Wang F, et al. Cardiovascular disease in early stages of chronic kidney disease in a Chinese population. J Am Soc Nephrol. 2006;17:2617-2621.
Ross Molinaro, PhD, MLS(ASCP)CM, DABCC, FACB serves as Head of Medical Affairs and Medical Officer for Siemens Healthcare Laboratory Diagnostics, Inc.
Carole Dauscher serves as Senior Global Marketing Manager for Siemens Healthcare Laboratory Diagnostics, Inc. providing marketing and business support for clinical chemistry and the continuum of diabetes and renal diagnostic testing.