Image is everything! Quality issues remain as digital pathology enters the clinical lab.
Digital microscope images are used in reports, papers, publications, posters, laboratory notebooks, and all kinds of presentations. They provide data on and insight into specimens, cases, and procedures. But how can a laboratory professional be sure that a digital microscope image is consistent and reliable? What assurance is there that any given equipment captures a true representation of the specimen or that a computer monitor adequately displays the image?
Are digital images really better?
Despite the convenience of digital imaging, the images sometimes fail to meet the expectations of clinicians, who rely on highly refined perceptive skills to identify features or uncover descriptive clues in specimen slides. Regardless of operator training and the quality of microscopes or cameras, captured images are not identical to the specimens they depict.1 Subtle variations in both equipment and operator behavior contribute to additional variation in images.
Nevertheless, digital imaging has become a standard practice in microscopy for research and teaching, and during the last several years, there has been a growing emphasis on the potential for digital microscopic images in clinical diagnostics in the United States. Digital pathology has grown in adoption and acceptance, as progress has been made in the validation and use of digital microscope images across a number of application areas.2-5 However, studies have revealed that current technology is not yet quite as good as the actual slide as viewed through a properly adjusted microscope. The old way is still viewed by most as “the gold standard.”
In one example, trained observers received pertinent case history and detail, but could review only digital images of patient specimens. Among 53 cases, observers achieved only a 91 percent concordance with original diagnoses made using a traditional microscope.6 Observers also could not identify cellular nuances in the digital images that were readily observed through the microscope. In another example, sample preparation was found to obscure specimen detail in digital images. However, when study participants used traditional microscopes, they were able to compensate for some of these anomalies, mostly by simple focus adjustments.7
Images may be inconsistent, even when they come from the same acquisition system,8 and image processing is generally accepted to facilitate evaluation, especially when such adjustments are documented and disclosed to viewers or readers.9,10 Evidence suggests that color correction and image consistency greatly improve the detection of tissue features for analysis.10-13 Regardless of the source of color variation, bringing consistency to color, contrast, and brightness allows ready evaluation of images and their comparison through automated analysis (i.e., segmentation and quantification based on tissue/cellular features). The accuracy of analysis depends on the consistency of the images, but color, a vital aspect of imaging, is notably complex. Images are impacted by the acquisition device, the acquisition and viewing software, and the devices upon which they are visualized.8,14
Color digital imaging challenges
Three key color challenges must be addressed for digital imaging to be successfully implemented in the laboratory.
The first is the creation of a standard to which color may be compared during the acquisition process; this standard must serve as the target toward which image correction is guided. Although several attempts have been made at creating color reference slides,14,16 their ability to deliver stable data is paramount, and almost all reference slides fade or decay over time.
The second is developing an easy-to-use interface that facilitates calibration and imaging control. Such an interface should integrate easily into existing acquisition or analysis software.
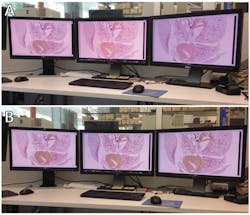
The third is the accurate display of images. Every observer, whether on a single workstation or within a network of linked viewers located across remote locations, should see the same thing. Today’s color monitors suffer from technological instabilities that result in color shift, contrast reduction, and a loss of the total viewable colors;15 therefore, what viewers see can be inconsistent (Figure 1).
Progress toward standards
In February 2015, the U.S. Food and Drug Administration (FDA) released draft guidance on verifying, controlling, and validating imaging system performance.17 Intended to address automated microscope slide scanners, also called whole slide imaging (WSI) devices, the draft guidance identifies opportunities to manage and control image acquisition in WSI devices. The document does not address the visualization of images beyond the computer monitor associated with the WSI scanner. (In other words, the guidance does not discuss how images are displayed and visualized for diagnosis.) Presumably, this topic will be addressed in another regulatory document that focuses on potential clinical application of WSI images.
Several industry organizations have been working to answer some of the dilemmas identified in the draft guidance, specifically regarding color performance in image acquisition and display. In May 2013, a working group was formed under the International Color Consortium (ICC) to provide manufacturer-independent advice regarding medical imaging with a focus on digital pathology and WSI. The Medical Imaging Working Group (ICC-MIWG) has convened on several occasions and drafted guidance on quality control throughout the WSI imaging workflow, from WSI device calibration and image data integrity validation, to display device calibration.
The ICC-MIWG and other organizations provided feedback to the FDA regarding the draft guidance, primarily in relation to the performance and testing of WSI devices. The FDA’s own WSI working group (FDA-WSIWG), which represents manufacturers and other industry authorities, regulatory experts, and physician/practitioners of pathology,18 also provided feedback, as did the Digital Pathology Association, which is comprised of pathologists, scientists, industry representatives, and technologists who seek to promote digital pathology imaging for education and training with a focus on defining best practices and influencing standards.
Regarding the first challenge of establishing standards for color image capture, the FDA draft guidance specifically addresses WSI, but its proposed controls and standards could be applied to digital microscope imaging in general, bringing greater consistency in images for research and analysis across disciplines and reaching far beyond pathology. Along with the specimen itself, light sources are the primary contributor to image color, whether viewed digitally or through the microscope. Over the past decade, there has been a transition toward light-emitting diode (LED) light sources, but specimen color rendering can be altered by LED light source configuration (i.e., 3-LEDs, commercial bright-white LED, color-temperature-balanced LED, etc.). In addition, there are still tens if not hundreds of thousands of microscopes that have halogen (tungsten filament) light sources. Microscope users will most definitely see variation in images depending on their light source.
Cameras, referred to as digital imaging sensors in the draft guidance, feature their own variances due to the devices’ physical characteristics and software features. Software typically manages exposure and white balance but may apply color correction at the discretion of the manufacturer. Since each manufacturer uses its own color profile, images are not consistent. This makes the second challenge of coming up with software interfaces that work across systems and devices even more complex.
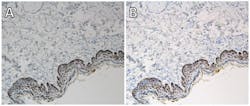
The third major challenge, visualization and display, is equally thorny. Even though computer monitors vary based on technology, and despite the deterioration of their color generation capability with age, displays can be calibrated using after-market devices or built-in calibration systems. To be useful, calibration must go far beyond the subjective adjustment of hue, saturation, tint, contrast, and brightness. True calibration uses a physical sensor (spectrophotometer or colorimeter) to measure the color-rendering capability of a monitor and then tune it to an industry standard. Researchers have found that calibrated monitors may help improve the speed and accuracy of digital image interpretation.19 The challenge for both WSI and imaging with traditional microscopes has been finding the tools (both standard slides and software) to manage image consistency and accuracy (Figure 2).
Stage micrometers, focusing targets, and resolution targets exist and are readily applied for WSI devices, but not necessarily for traditional microscopes. Several groups have attempted to develop a color calibration target for WSI, generally involving either colored plastic strips on a microscope slide14,16 or photographic film, but the color targets have been too large to be imaged on a traditional microscope. Calibration has been imperfectly integrated into the systems, often requiring the services of a field service engineer or a graduate student with a propensity for writing computational algorithms.
One combination of a calibration slide and software is commercially available. The slide features color patches that are not susceptible to fading like film or biological-based slides, and the associated software applies color management principles to correct images for color and brightness, regardless of the microscope used to capture them. The system’s monitor calibration software uses a colorimeter to ensure that images are displayed consistently.
Just around the bend
At the end of this year, the FDA plans to release the final guidance for WSI devices being used for digital pathology. At that time, WSI device manufacturers will address validation requirements. But WSI for digital pathology is just one use of digital images. Digital pathology also can be performed using traditional microscopes with digital cameras, and these instruments are beyond the FDA’s current guidance. However, as the FDA is setting the standard for image quality and consistency for clinical use, the digital microscope imaging field will expect, even demand, that microscope and camera manufacturers, system integrators and image analysis software providers, embrace the same standards for quality and performance as outlined by the FDA. Until that time, laboratory professionals will continue to use the tools and skills available to manage their own images and imaging systems for quality and consistency.
References
- Pantanowitz L. Digital images and the future of digital pathology. J Digital Imag. 2010;1(1): 15.
- Brick KE, Sluzevich JC, Cappel MA, DiCaudo DJ, Comfere NI, Wieland CN. Comparison of virtual microscopy and glass slide microscopy among dermatology residents during a simulated in‐training examination.” J Cutaneous Pathol.2013;40(9):807-811.
- Costello SS, Johnston DJ, Dervan PA, O’Shea DG. Development and evaluation of the virtual pathology slide: a new tool in telepathology.” J Med Internet Res. 2003;5(2):e11.
- Gilbertson JR, Ho J, Anthony L, Jukic DM, Yagi Y, Parwani AV. Primary histologic diagnosis using automated whole slide imaging: a validation study. BMC Clin Pathol. 2006;6(1):4.
- Sanders DSA, Grabsch H, Harrison R, et al. Comparing virtual with conventional microscopy for the consensus diagnosis of Barrett’s neoplasia in the AspECT Barrett’s chemoprevention trial pathology audit. Histopathology. 2012;61(5):795-800.
- Wilbur DC, Madi K, Colvin RB, et al. Whole-slide imaging digital pathology as a platform for teleconsultation: a pilot study using paired subspecialist correlations. Arch Pathol & Lab Med. 2009;133(12):1949.
- Gavrielides MA, Conway C, O’Flaherty N, Gallas BD, Hewitt SM. Observer performance in the use of digital and optical microscopy for the interpretation of tissue-based biomarkers. Analytical Cell Pathol. 2014;2014:157308. http://dx.doi.org/10.1155/2014/157308. Accessed July 25, 2015.
- Badano A, Revie C, Casertano, et al. Consistency and standardization of color in medical imaging: a consensus report. J Digital Imag. 2014;28(1):41-52.
- Murakami Y, Abe T, Hashiguchi A, Yamaguchi M, Saito A, Sakamoto M. Color correction for automatic fibrosis quantification in liver biopsy specimens. J Pathol Informatics. 2013;4(36). http://doi:10.4103/2153-3539.124009. Accessed July 25, 2015.
- Sedgewick J. Scientific imaging with Photoshop: methods, measurement, and output. Peachpit Press, 2010.
- Hackett EJ, Amsterdamska O, Lynch M, Wajcman, J. The handbook of science and technology studies. The MIT Press. 2008.
- Murakami Y, Abe T, Yamashita Y et al. Color processing in pathology image analysis system for liver biopsy. 22nd Color and Imaging Conference Final Program and Proceedings and 2nd Congress of the International Academy of Digital Pathology 2014;184-188.
- Linden MA, Sedgewick GJ, Ericson M. An innovative method for obtaining consistent images and quantification of histochemically stained specimens. J Histochem Cytochem. 2015;63(4):233-243.
- Yagi, Y. “Color standardization and optimization in whole slide imaging.” Diag Pathol. 2011;6(Suppl 1):S15.
- Cheng WC, Keay T, O’Flaherty, et al. Assessing color reproducibility of whole-slide imaging scanners. Proceedings SPIE Medical Imaging. International Society for Optics and Photonics. 2013;86760O.
- Revie C, Orf D, Davis G, Sweeney J, et al. Whole slide imaging. International Color Consortium: Medical Imaging Working Group Minutes. January 2015. http://www.color.org/groups/medical/wsi/Minutes_Jan_2015_Calibration-Slide-Histopath.pdf. Accessed July 25, 2015.
- U.S. Department of Health and Human Services, Food and Drug Administration, Center for Devices and Radiological Health. Technical performance assessment of digital pathology whole slide imaging devices: draft guidance for industry and food and drug administration staff.” February 25, 2015. http://www.fda.gov/ucm/groups/fdagov-public/@fdagov-meddev-gen/documents/document/ucm435355.pdf. Accessed July 25, 2015.
- Treanor D, Gallas BD, Gavrielides MA, Hewitt SM. Evaluating whole slide imaging: a working group opportunity.” J Pathol Informatics. 2015;6:4.
- Krupinski EA, Silverstein LD, Hashmi SF, Graham AR, Weinstein RS, Roehrig H. Observer performance using virtual pathology slides: impact of LCD color reproduction accuracy.” J Digital Imag. 2012;25(6), 738-743.