The utility of glycosylated hemoglobin measurements in the management of patients with diabetes has been established for almost 40 years.1-6 Hemoglobin A1c (HbA1c) is the most abundant minor hemoglobin in normal red cells and is elevated as much as threefold in people with diabetes. The term, “A1c” should be reserved for HbA, which has glucose attached to the N-terminus of the b-chain by a ketoamine linkage. Glucose is also linked in the same way to other sites of the hemoglobin molecule such as the N-terminus of the a-chain and certain lysine residues. In diabetic patients these glucose adducts are increased in parallel with HbA1c. HbA1c is formed slowly and continuously throughout the life of the red cell. Thus HbA1c levels should provide an integrated assessment of blood glucose levels over the preceding 90 to 120 days.
Glycated hemoglobin: strengths and limitations
Initially high-performance liquid chromatographie (HPLC) ion exchange and affinity chromatography mini-columns were primarily employed in most clinical laboratories as methodologies for measuring A1c. A comparison of the determination of glycosolated hemoglobin by affinity chromatography to ion-exchange methods has been published.7 The authors reported that, although the methods correlated well, they observed that the affinity technique showed higher percentages of glycated hemoglobins than would be obtained by using ion-change chromatography. The affinity support binds a greater range of glycated species, leading to the conclusion that affinity methods may more accurately represent the levels of glycosylation. (Methods of obtaining glycated hemoglobin values are expressed in detail in Table 1.)
An automated POC immunoassay (Bayer DCA 2000, now Siemens) for glycated hemoglobin was developed and evaluated in the early 90s.8-10 One evaluation of the Siemens DCA 2000 concluded that when compared to an HPLC method it had significant constant and proportional values.11 At low concentrations of hemoglobin F the DCA 2000 underestimated HbA1c and at higher hemoglobin F concentrations tended to overestimate HbA1c. Recently the DCA vantage offered an improvement over the previous version and passed the National Glycohemoglobin Standardization Program (NGSP) criteria for acceptable performance. Later, immunoassays were also developed for automated multichannel chemistry analyzers, including the Abbott Architect/Aeroset, Siemens Dimension and Advia, Beckman Unicel DxC/Synchron and AU, Roche Cobas Integra and Ortho Clinical Diagnostics Vitros. Immunoassays quantify HbA1c by using antibodies that recognize the structure of the N-terminal glycated amino acids on the b chain of Hb A.While many laboratories favored the affinity approach because it measured all glycated fractions, it became obvious that the two methods were producing different results, leading to two distinct normal ranges. This complicated the interpretation of results among different medical centers, which became more complex with the implementation of the Diabetes Control and Complications Trial (DCCT). The 29 centers involved sent samples from patients in the study to a central location at the Joslin Diabetes Center in Massachusetts. Ion-exchange HPLC was chosen as the method for the analysis of all samples. The study demonstrated the utility of measuring HbA1c to monitor and treat people with diabetes, negating any perceived advantage of determining total glycated hemoglobin. Since ion-exchange HPLC was used in the DCCT study, it became widely accepted and is commonly used for HbA1c measurement today.
First-generation assay epitopes typically span amino acids 4 to 10 of the Hb b chain, encompassing the altered amino acid at position 6 present in Hb S and Hb C variants. HbA1c measurement interferences in the presence of these Hb variants prompted the development of second-generation assays that used antibodies developed to epitopes spanning amino acids 1 to 4. Second-generation assay antibodies largely eliminated analytic interferences from Hb S and Hb C and improved the overall accuracy when compared to first-generation assays. The second-generation assay antibodies are thought to bind Hb S and Hb C variants similarly to Hb A, and thus provide clinically useful HbA1c values for patients with heterozygous trait conditions, where red blood cell life span is thought to be essentially normal.
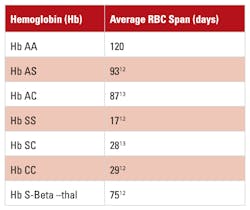
However, as shown in Table 2 this assumption is not always valid. In addition, these assays create an increased risk for reporting misleading HbA1c results for patients harboring homozygous Hb S, Hb SC, or Hb S–b-thalassemia, where red blood cell life span is decreased. These assays provide an ostensibly reportable HbA1c value without warning that the patients appeared to not have any Hb A. Although the values labeled as HbA1c, obtained by using these methods, may be fairly accurate, their clinical significance may be misleading and do not reflect glycemic status accurately. The ADA recommends against the use of HbA1c for patients with certain Hb variants, such as Hb SS, Hb SC, CC and Hb S–b-thalassemia. The HPLC affinity method can also be misleading because it also provides an HbA1c in the absence of Hb A. Table 1 illustrates how ion exchange, immunoassay, and affinity chromatography are affected by the presence of homozygous and compound heterozygous variants. It also indicates that ion exchange methods can provide obviously erroneous results in the presence of the variants.
Methods that detect other glycated fractions report an HbA1c value even though there is no hemoglobin A. Table 2 illustrates the variation of red cell half-life when homozygous, double or compound heterozygous, and heterozygous variants are present. Even heterozygous variants such as AS or AC have reduced half-lives. It is assumed that the shortened red cell life in such hemoglobins does not affect glycation rates, but questions have been raised about whether that is always true.
NGSP and standardization
The purpose of the NGSP is to standardize glycohemoglobin test results (HbA1c) so that clinical laboratory results are comparable to those reported in the DCCT where relationships to mean blood glucose and risk for vascular complications have been established. The methods and reagents are certified by the NGSP as having documented traceability to the DCCT reference method. Currently, 16 pages of methods and instruments are certified, encompassing more than 200 different combinations of instruments and reagents. The certificate of traceability expires after one year, and the NGSP recommends that manufacturers certify their methods yearly. The most recent certifications occurred in April 2015.
Testing for diabetes with HbA1c
To meet tightening College of American Pathologists (CAP) requirements and to provide accurate HbA1c medical decision information to physicians and patients, laboratories will need to use an HbA1c assay that is accurate and precise. The information on the performance of individual methods can be easily obtained by reviewing CAP GH2 Summary reports or from the NGSP website. In addition to greater accuracy and precision, it is important to use an HbA1c method that will identify abnormal hemoglobins that could impact HbA1c results. Improvements in methodologies used in regard to precision, accuracy, and the ability to detect Hb variants are preparing the market for a shift toward diabetes screening with HbA1c.
In January 2010, the American Diabetes Association (ADA) announced its long-awaited recommendations for diagnosis of diabetes. According to recommendations, testing for diabetes should be considered for all adults who are overweight (BMI 25 kg/m2) and have additional risk factors. The ADA established the threshold of diabetes to be ≥6.5% HbA1c. The HbA1c test should be performed using a method certified by NGSP and standardized to DCCT reference study. An HbA1c range of 5.7-6.4% identified individuals with a high risk for future or pre-diabetes. POC HbA1c assays are not sufficiently accurate at this time to use for diagnostic purposes.
For patients with Hb variants but normal red blood count (RBC) turnover, HbA1c assays without inferences from abnormal hemoglobins can be used. The problem is that the laboratory may not have the patient history indicating what Hb variant is present. As mentioned earlier, affinity and immunoassay methods cannot detect any of the variants. The NGSP website summarizes the effects of hemoglobin variants and elevated fetal hemoglobin on methods and instruments for HbA1c.15
Individualized Quality Control Plan
New quality control recommendations by Centers for Medicare and Medicaid Services (CMS) allow clinical laboratories to develop an Individualized Quality Control Plan (IQCP) based on risk management. In a recent report,19 sigma-metric values (total allowable error- %bias)/CV) were calculated for some commonly used HbA1c techniques. Sigma-metric directly relates to the predicted probability of producing an unreliable patient result; higher sigma-metric indicates better method performance. In CAP surveys, the total allowable error is currently set at 6% (previously, at 7%; it is projected to be brought down to 5% in the near future).
With the initiative to use glycated hemoglobin to test for diabetes, it is even more imperative that the assay for this analyte is accurate and precise. Manufacturers of testing that involves ion exchange and capillary zone electrophoresis claim that only these techniques actually measure glycation at the N-terminus of the beta chain; other methods, they assert, also include other glycated hemoglobins in the reported results, and in the presence of hemoglobin variants immunoassays and affinity methods may produce misleading results. Precision and accuracy are also extremely important, since the ADA has recommended any value above 6.5 as diagnostic for diabetes. Laboratories must carefully choose instruments and methods to perform HbA1c testing and have a clear understanding of what is really being measured. Finally, it is imperative that the laboratory effectively communicate the limitations of their method to care givers.
References
- Bunn HF, Evaluation of glycosylated hemoglobin in diabetic patients.Diabetes. 1981; 30:613-617.
- Koenig RJ., Peterson CM, Jones, RL, et al. Correlation of glucose regulation and hemoglobin A,o in diabetes mellitus. NEJM. 1976;295:417-420.
- Gabbay KH, Hasty K, Breslow JL, et al. Glycosylated hemoglobins and long-term blood glucose control in diabetes mellitus. J Clin Endocrinol Metab. 1977;44:859-864.
- Gonen G, Rubenstein AH, Rochman H, Tanega SP, Horwitz D L. Haemoglobin A. an indication of the metabolic control of diabetic patients. Lancet 1977;2:734-737.
- Dolhofer R, Stadele K, Wieland, OK. Clinical and biochemical studies on the significance and formation of hemoglobins A1e and A1a+b in diabetes mellitus. Klin Wochenschr. 1977;55:945-954.
- Graf RJ, Halter JB, Porte D. Glycosylated hemoglobin in normal subjects and subjects with maturity-onset diabetes. Diabetes. 1977;27:834-839.
- Klenk DC, Hermanson GT, Krohn RI, et al. Determination of glycosolated hemoglobin by affinity chromatography: Comparison with colorimetric and ion-exchange methods, and effects of common interferences. Clin Chem. 1982;28:2088-2094.
- Ng RH, Sparks KM, Hiar CE. Rapid automated immunoassay system measuring hemoglobin A1c by using precalibrated, unitized reagent cartridges.Clin Chem. 1992;38;1647.
- Guthrie R, Hellman R, Fineberg NS, et al. A multi physician's office laboratory evaluation of an immunological method for the measurement of HbA1c, Diabetes Care.1992;15:1494-1498.
- Marrero DG, Vandagriff JL, Gibson R, et al. Immediate HbA1c results , Performance of new HbA1c system in pediatric outpatient population, Diabetes Care,.1992;15:1045-1049.
- Diem P, Walchli M, Primus ME, Marti U. Agreement between HbA1c measured by DCA 2000 and by HPLC: effects of fetal hemoglobin concentrations. Archives of Medical Research. 2004;35:145-149.
- McCurdy PR. 32-DFP and 51-Cr for measurement of red cell life span in abnormal hemoglobin syndromes, Blood.1969;33(2):214-224.
- Prindle KH, McCurdy PR. Red cell lifespan in hemoglobin C disorders (with special reference to hemoglobin C trait), Blood. 1970:36:14-19.
- McCurdy PR, Mahmood L, Sherman AS., Red cell life span in sickle cell-hemoglobin C disease with a note about sickle cell-hemoglobin O ARAB, Blood. 1975; 45(2):273-279.
- NGSPwebsite. www.ngsp.org. Accessed May 7, 2015.
- Rhea JM, Koch D, Ritchie J, et al. Unintended reporting of misleading HbA1c values when using assays incapable of detecting hemoglobin variants. Arch Pathol Lab Med. 2013; 137:P1788-1791.
- Rhea JM, Koch D, Ritchie J, et al. Unintended reporting of misleading HbA1c values when using assays incapable of detecting hemoglobin variants. Arch Pathol Lab Med. 2013;137:P1788-1791.
- Lenters-Westra E, Slingerland RJ. Three of seven hemglobin A1c point-of-care instruments do not meet generally accepted analytical performance criteria, Clin Chem. 2014;60(8 ):1062.
- Woodworth A, Korpi-Steiner N, Miller, JJ, et al. Utilization of assay performance Characteristics to estimate hemoglobin A1c result reliability. Clin Chem. 2014;60:1073-1079.