Bringing down barriers to give better access to mass spectrometry in the clinical laboratory
Have you ever wanted to either implement or expand the use of liquid chromatography – tandem mass spectrometry (LC-MS/MS) in your clinical lab but have put off doing so because of the apparent complexity and expertise needed? You’re not alone. Optimal application and expansion of LC-MS/MS in the clinical laboratory can bring a number of benefits, but that expansion depends on overcoming several long-standing barriers.
Benefits of LC-MS/MS
LC-MS/MS has long been recognized as an important and powerful measurement technology due to its enhanced specificity, accuracy, and precision as compared to immunoassays.1 It is able to address various cross-reactivities or interferences commonly seen with other methodologies, and for the quantification of small molecules in human sample materials, it is a particularly good solution.2 The most prominent applications and indeed benefits for clinical LC-MS/MS in measuring and reporting patient results are for small molecule analytes in the areas of endocrinology, hormone measurements, immunosuppressant, and therapeutic drug monitoring and drugs of abuse testing. There is little argument that these techniques provide a higher level of sensitivity and specificity for many analytes compared to other analytical techniques and that patient care has benefited from its use.1
25-hydroxyvitamin D (25(OH)D) provides good examples of the benefits of enhanced analytical and clinical quality of LC-MS/MS. Under-recovery of the 25(OH)D2 component of total vitamin D, and cross-reactivity of the C3-epimer forms of 25(OH)D, as well as cross-reactivity of the (24,25(OH)2D metabolites with various automated immunoassays are 3 meaningful examples that have been studied.3-9 In the field of neonates and infants, those needing to have their vitamin D status monitored showed mean concentrations of the C3-epimer of between 10% to 61% of the total Vitamin D concentration.5
Assays for immunosuppressant drugs, which require close monitoring of patient drug concentrations for several reasons, offer another good example of the general accuracy advantages of LC-MS/MS. The bias of the results reported for different immunoassays compared to LC-MS/MS procedure varies widely. Immunoassays can exhibit interferences due to cross-reactivity with other drugs and metabolites, reactions with heterophilic antibodies, antibodies directed against the binding antibody, and the effects of endogenous factors such as hematocrit, albumin, bilirubin, and triglycerides.10 The structure of immunosuppressant drug metabolites is similar to the parent drug; therefore, it is difficult if not impossible to construct an immunoassay that recognizes the parent compound without some degree of cross-reactivity towards one or more of the metabolites. LC-MS/MS assays have been developed for immunosuppressants and are widely used in clinical practice. The chromatographic conditions can be established such that none of the metabolites interfere with the quantitation of the parent drug in the assay.11
Barriers to LC-MS/MS use
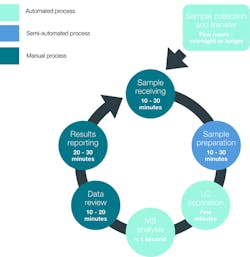
Despite these benefits, LC-MS/MS has been inaccessible for many clinical labs and, for labs that have implemented it, challenging to expand and optimize its utilization. Current mass spectrometry practice does not necessarily fit the overall workflow in a clinical laboratory.12 It involves multiple manual steps and batch processing, and therefore, assuring consistency between instruments over time can be problematic (Figure 1). Multiple instruments and software need to be individually acquired and assessed and put together as connected components. The throughput is lower than other chemistry or immunoassay analyzers in clinical laboratories, which lessens productivity and increases turnaround time for many clinical applications.12 Assay methods are often lab-developed tests (LDTs) whose development and implementation can be challenging. To establish accurate and reproducible MS-based LDTs, a laboratory requires a significant level of expertise.
Consequently, LC-MS/MS has not gained traction in hospital laboratories as much as had been anticipated a decade ago.13 When asked to identify the key challenges hospital laboratories are facing, staffing (26%) was identified as the top priority.14 The Bureau of Labor Statistics has projected a nationwide increase in demand for medical and clinical laboratory technologist of 13% between 2016 and 2026, while at the same time current vacancy rates remain high, averaging 7.2%.15 Limited access to this kind of expertise and extensive technical training requirements has hindered further growth and implementation of this platform in the clinical lab.12
Automation of LC-MS/MS in clinical laboratories
It’s broadly accepted that advances in automation will be paramount to break through this bottleneck and increase the appeal for LC-MS/MS in routine use.12 The next level of automation has to aim at system solutions with features of practicability that are similar to those of today’s immunoassay systems and can be operated by ordinarily trained technicians. Progress toward such solutions will make the analytical powers of LC-MS/MS useful for clinical medicine and will reduce the barriers of entry, increase scalability, and enable the LC-MS/MS platform to be accessible and implemented in routine clinical laboratories.2,12 While it will maximize walk-away time and increase throughput, total automation requires other considerations such as a broad test menu, the availability of commercial assay kits, standardization and results harmonization, and timely technical services.12 Key specifications of a future plug-and-play LC-MS/MS–based clinical analyzer system should include the following elements:2,12
- Random access /multichannel mode
- Handling of standard clinical laboratory sample tubes
- Bar code reading of tubes for positive identification – bi-directional interface to local LIMS
- Complete and closed management of ready-made solvents
- Ready-to-use and bar code identified internal standards
- Comprehensive auto-start, auto-tuning, auto-validation, and auto-QC functions
- Comprehensive control of all subsystems
- Auto-maintenance feature
- Flexible equipment acquisition models beyond just capital purchase options
- 24-hour technical support and short turnaround time service will be essential, for smooth operation and to minimize downtime.
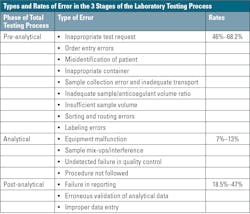
Such solutions of automation are helpful to increase throughput, avoid sources of gross errors, increase the productivity and reliability of analyses, and reduce hands-on time of skilled technicians.2 Overcoming challenges with staffing, fluctuations in testing volumes, improving turnaround times, and reducing errors and costs are all proven benefits that have fueled the adoption of laboratory automation. The potential for error reduction is an important benefit of these types of solutions (Figure 2). Published data suggest that 24% – 30% of laboratory errors influence patient care, while actual or potential patient harm occurs in 3% – 12% of cases.15
Conclusion
Indeed LC-MS/MS can close substantial gaps in the parameter portfolio of laboratory medicine by allowing routine analyses on a reference method level of accuracy and by facilitating comprehensive testing panels.2 While LC-MS/MS is making its way into many clinical laboratories, it is still limited to specialty laboratories and many challenges prevent it from being implemented routinely.12 The ability to offer LC-MS/MS in a random-access high-volume analyzer will improve both turnaround times, as well as improve sensitivity and specificity of the assays.13
But, going forward, will fully automated IVD platforms become the only way LC-MS/MS testing is implemented in the clinical lab? It’s unlikely that will be the case. In 2019, a small survey of hospital LC-MS laboratory directors were asked whether they would transition all clinical mass spectrometry testing to a closed, fully automated Food and Drug Administration (FDA)-approved LC-MS/MS system if one became available — only one respondent would choose replacement. 13 More than half said they would retain their open-access, lab-developed LC-MS/MS tests and continue to develop additional LDTs, as well as using a fully automated closed LC-MS/MS system.
In the near future, MS platforms may be differentiated into two categories: one would be the current existing system for esoteric and low-volume laboratory developed tests; the second category would be a basic model sold as a medical device for high-volume and well-defined tests such as vitamin D, drugs of abuse screening and confirmation, and immunosuppressant drugs. The latter category may achieve higher-level automation and standardization for routine clinical usage.12 In addition, with fully automated, total-solution LC-MS/MS clinical analyzers coming into the picture, it will open the door for new acquisition options such as reagent rental or cost per reportable result (CPRR), which will further facilitate clinical LC-MS/MS adoption. And, in some cases both fully automated, closed systems and the current existing systems for esoteric and low-volume LDTs may be offered under a single, combined CPRR arrangement, further enabling optimization.
Technological advancements in the available options for clinical mass spectrometry are progressing at a rapid pace. Leveraging the continuum of LC-MS/MS technologies (open systems for LDTs, including Class I medical devices and fully automated Class II IVD analyzer systems) will be the path forward for expansion and optimization of this powerful measurement technology for the clinical lab.
References
- Hammett-Stabler, et. al., The Evolution of Mass Spectrometry in the Clinical Laboratory, Clinical Applications of Mass Spectrometry, Methods in Molecular Biology 603, Chapter 1, Humana Press, 2010.
- Vogesor, et. al., Progress in automation of LC-MS in laboratory medicine, Clinical Biochemistry, 44 (2011) 4-13.
- Freeman et al. Performance evaluation of four 25-hydroxyvitamin D assays to measure 25-hydroxyvitamin D2. Clin Biochem. 2015; 48(16–17):1097-1104.
- Tolan et al. Price of High-Throughput 25-Hydroxyvitamin D Immunoassays: Frequency of Inaccurate Results. J App Lab Med. 2018; 2(6): 868-879.
- Erdman et al. Accuracy-Based Vitamin D Survey: Six Years of Quality Improvement Guided by Proficiency Testing. Arch Path Lab Med. 2019; 143(12): 1531-1538.
- Bailey et al. Analytical measurement and clinical relevance of vitamin D3 C3-epimer. Clin Biochem. 2013; 46(3): 190-196.
- Singh et al. C-3 Epimers Can Account for a Significant Proportion of Total Circulating 25-Hydroxyvitamin D in Infants, Complicating Accurate Measurement and Interpretation of Vitamin D Status. J Clin Endocrinol Metab. 2006; 91(8): 3055-3061.
- Cashman et al. Significance of Serum 24,25-Dihydroxyvitamin D in the Assessment of Vitamin D Status: A Double-edged Sword? Clin Chem 2015; 61(4): 636-645.
- Bosworth et al. The serum 24, 25-dihydroxyvitamin D concentration, a marker of vitamin D catabolism, is reduced in chronic kidney disease. Kidney Int 2012; 82: 693-700.
- Pierre Wallemacq, et. al. Assuring the Proper Analytical Performance of Measurement Procedures for Immunosuppressive Drug Concentrations in Clinical Practice: Recommendations of the International Association of Therapeutic Drug Monitoring and Clinical Toxicology Immunosuppressive Drug Scientific Committee, Ther Drug Monit, Vol 00, Number 00, Month 2016.
- Alan Wu, Deborah French, et. al., Implementation of liquid chromatography/mass spectrometry into the clinical laboratory, Clinica Chemica Acta 420 (2013) 4-10.
- Yan Victoria Zhang and Alan Rockwood, Impact of automation on mass spectrometry, Clinica Chimica Acta, 450 (2015) 298 – 303.
- Kimberly Scott, The Road to Automation in Clinical Mass Spectrometry, Clinical Laboratory News (CLN), September 2020, pgs. 12 – 14.
- The benefits of lab automation to facilitate testing for SARS-CoV-2, Jamie Gramz, BSE, MBA, MLO, December 2020.
- Minimizing laboratory errors with automation, By Jacqui Reithel, MBA, MT (ASCP), MLO, August 2021.
Mikko Salonen, System R&D Director, Clinical Mass Spectrometry, Thermo Fisher Scientific.
Peter Cooke, Americas Regional Marketing Manager, Clinical Mass Spectrometry, Thermo Fisher.
About the Author

Mikko Salonen
System R&D Director, Clinical Mass Spectrometry, Thermo Fisher Scientific.

Peter Cooke
Americas Regional Marketing Manager, Clinical Mass Spectrometry, Thermo Fisher Scientific.