Since the early days of the COVID-19 pandemic, labs, and other healthcare settings, have used molecular and antigen diagnostic tests to detect SARS-CoV-2, the virus that causes COVID-19, in patients’ specimens.
Then came Omicron. After the variant’s arrival in late 2021, public health and government officials began to analyze the impact of Omicron on test results.
The concern centers around the number of mutations in Omicron, compared with earlier variants and the original virus. Many of these occur in the spike protein, which the Centers for Disease Control and Prevention (CDC) said, “is characterized by at least 30 amino acid substitutions, three small deletions, and one small insertion. Notably, 15 of the 30 amino acid substitutions are in the receptor binding domain (RBD). There are also a number of changes and deletions in other genomic regions.”
Experts say Omicron is more easily spread among people than earlier versions of the virus. This has been confirmed by the World Health Organization (WHO), where Omicron’s overall risk remains classified as “very high risk.” Indeed, the Centers for Disease Control and Prevention (CDC) reported recently that Omicron now accounts for 95% of COVID-19 cases in the United States, compared with 5% for Delta.
The WHO also said that “the rapid growth rate is likely to be a combination of both immune evasion and intrinsic increased transmissibility of the Omicron variant.”
Upon investigation, what seems to be significant with testing for SARS-CoV-2, when the Omicron variant is present, is two-fold.
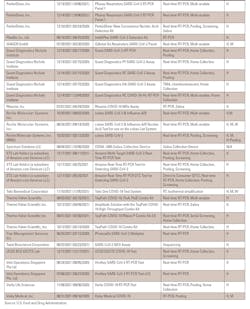
First, there is the potential impact on molecular tests. The Food and Drug Administration (FDA) has published lists of diagnostic tests that have been potentially impacted by SARS-CoV-2 variants. The issue is whether a diagnostic test will produce accurate results if a mutation occurs in the virus in the gene target that the test is designed to detect. If a test detects multiple gene targets, it will still detect the presence of SARS-CoV-2 via the other targets and produce accurate test results, the FDA said.
However, if the test is a single-target or multiple-target assay in which all of its targets are impacted by mutations, it will fail to detect Omicron, leading to false negative test results. The FDA has created a list of such tests.
Originally, the DTPM COVID-19 RT-PCR test from Tide Laboratories was on the list. However, the manufacturer has since modified the test, and the FDA reissued an emergency use authorization (EUA) for the test in late December 2021.
“The original test was a single target test that was expected to fail to detect the SARS-CoV-2 Omicron variant (B.1.1.529) due to a nine-nucleotide deletion in the N-gene, spanning positions 28370-28362, which is within the portion of the N-gene that the single target covered,” the FDA explained. However, the manufacturer has since modified the test, which is now a multiplex test with an added reverse primer to detect the Omicron variant.
A bioinformatics analysis demonstrated a 100% match with Omicron and Delta variant sequences, the FDA said.
Several other tests remain on the list of tests expected to fail to detect Omicron.
The FDA noted that other tests may have one genetic target, with reduced sensitivity, due to a mutation in the Omicron variant related to the S gene, but test results should not be impacted because the tests detect multiple genetic targets in the virus. Those tests are included in a list of “Tests with Detection Patterns that May Be Associated with the SARS-CoV-2 Omicron Variant.”
The agency also said some of these may have an unintended benefit: the tests may signal that the Omicron variant is present in a sample, meaning that these specimens should be prioritized for sequencing.
Examples of those types of tests are Thermo Fisher Scientific’s PCR TaqPath COVID-19 Combo Kit and TaqPath COVID-19 CE-IVD RT-PCR Kit. The tests detect SARS-CoV-2 infections “by identifying the presence of three gene targets from the orf1a/b, S, and N regions of the virus. By surveying across multiple genes, the test can report accurate results even in the case where one of the targets (the S gene target) is impacted by a mutation,” the company said. It added that ”the overall accuracy of the TaqPath COVID-19 assays is not impacted.” Thermo Fisher has been touting its tests as useful for providing an early indication of the possible presence of Omicron in a sample.
Second, antigen tests may be less sensitive to Omicron than molecular PCR tests. Preliminary data, from the FDA suggests that antigen tests detect Omicron, but may be less sensitive. The FDA updated the SARS-CoV-2 Viral Mutations: Impact on COVID-19 Tests web page to share new information about the impact of the SARS-CoV-2 Omicron variant on antigen diagnostic tests. The update includes preliminary study results of some antigen tests using patient samples containing live virus.
In addition to the FDA’s research, a small real-world study by the COVID Sports and Society Workgroup finds antigen tests fail to detect Omicron during early days of infection. The study found that the median time, from the first positive PCR test to the first positive antigen test, was three days.