State of the Industry for molecular diagnostics in 2021
In the year that has passed since the first State of the Industry (SOI) on molecular diagnostics, a survey that Medical Laboratory Observer sent out to its readership of laboratory professionals, there have been many changes. With the influence of the pandemic, MLO asked laboratorians if they were still responding to supply shortages, had purchased new equipment, or made any relevant changes in the area of molecular diagnostics in the lab.
The results of this year’s SOI are in, and while various shortages surprised healthcare professionals, it seems the pandemic fueled innovation, as the U.S. Food and Drug Administration (FDA) granted emergency use authorizations (EUAs) to a number of tests that hit the market. Labs received training, and in some cases, reverted back to manual methods when automated components ran short.
Coordinating testing solutions
The demand for technology increased during the pandemic, especially for tracking the virus and variants, with labs across the globe sharing their viral data. When labs were asked how their facility handled reporting COVID-19 test results to the various authorities, 69% said they use specific software for reporting results, while 30% send aggregate test results with only the data requested, and 15% send aggregate test results with all data to be reviewed.
Only half of labs surveyed were tracking COVID-19 results. Of the labs tracking the virus, 26% were only tracking positive and negative COVID-19 test results, 12% had another facility tracking their results, 8% were tracking COVID-19 using genomic sequencing to monitor variants, 3% used to track COVID-19 but have since stopped tracking the virus, and only 1% were tracking COVID-19 and variants.
During the months when labs thought COVID-19 might be starting to wane, Ranalli remarked how many labs were expanding in-house testing options with the equipment purchased for COVID-19.
When labs were asked if they had excess capacity with analyzers that they had purchased to handle COVID-19 testing, results were split, with 47% replying yes, and 53% replying no.
Of the respondents who said they had excess capacity in analyzers purchased for the pandemic, more than half, 51%, planned to add new tests in-house, and 12% wanted to retire some analyzers. “Other” responses included donating the equipment to a mission, reallocating it to other departments, or using it for testing at schools and events. Some respondents simply planned to remain prepared, a smart move after new variants caused an influx of additional testing.
Ranalli, familiar with the seasonal changes and challenges in diagnostics, especially during respiratory season, discussed how some products, such as flu assays, will fluctuate in demand with the seasons, sometimes unpredictably, such as with the Delta variant.
Training during the pandemic
As social distancing during the pandemic influenced training, Ranalli shared how many field specialists turned to training via Zoom or video chat, before they were able to go to facilities in person. Charting unpredictable territory with unexpected waves of changing needs and supply availability issues, labs turned to technology for troubleshooting and training, she said.
On-demand videos helped labs with staff turnover, or when the third shift was not getting the same training as the morning shift. “This opened the channel for communication, refresher courses online and recorded videos, as some labs were using skills they had not used in a while, sometimes out of necessity due to shortages or other issues.” Ranalli compared it to “students getting out of school for the summer and needing a refresher in the fall.”
Supply shortages in the lab
The SOI survey also asked if labs had issues maintaining a supply of testing products for COVID-19. More than half, 58%, replied yes, and 42% replied no, they have adequate supply. As for non-COVID-19 products, 70% said they are lacking, while 30% said no, supply is adequate.
Swabs and consumables topped the list of supplies hard to secure, with 47% of labs feeling the shortage. Testing kits for SARS-CoV-2 came in at a close second, 42%; followed by pipettes, 36%; molecular tests, 29%; transport media, 29%; test tubes, 26%; controls/reagents, 21%; and personal protective equipment (PPE), 20%.
Labs listed “other” responses: hematology and media items, histology supplies, serology kits, cryovials, syringes, pipette tips and plastics, like bottles; 13% of labs reported no shortages.
The SOI survey revealed 87% of labs had shortages in 2020. Test kits topped the list,76%; swabs and consumables, 74%; transport media, 66%; PPE shortages, 44%; and controls/reagents, 32%.
Test choice based on availability
The MLO SOI survey also asked labs which of the common methodologies they used for COVID-19 testing; 84% responded they only used commercial test kits with an EUA, while 9% used lab-developed tests, and 7% used a combination of EUA and lab-developed tests.
When asked why they chose a particular method, 42% said it was due to test availability, while 34% looked at accuracy and reliability, 9% considered the cost of the test, and 6% considered turnaround time (TAT). Other lab responders said they already had analyzers in place, some were provider-driven choices, and others had an open platform.
Diagnosing at the molecular level
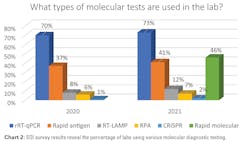
When polled about the types of molecular diagnostic tests used by labs to detect COVID-19, 73% said they used reverse transcriptase quantitative polymerase chain reaction (rRT-qPCR). Rapid molecular tests were next at 46%, followed by rapid antigen tests, 41%; reverse transcription loop-mediated isothermal amplification (RT-LAMP), 12%; recombinase polymerase amplification (RPA), 7%; and CRISPR-based diagnostics, 2%.
He added, “Mass spectrometry has been heavily pursued as an alternative supply chain to PCR, which was stretched at the height of the pandemic. Since COVID-19 testing reduced the usage of those instruments for their traditional tests, a capacity opportunity presented itself for the increase in its utilization. COVID MS-based assays were primarily focused on the identification of the nucleocapsid protein; with proteins being stable analytes, this makes for an attractive attribute for a robust clinical assay.” Preparation costs are similar to PCR reagents.
Improving test quality
With new technologies and when testing high volumes, errors can happen. How do labs handle questionable results with molecular tests? A majority, or 62%, of this year’s facilities opt to repeat the test with either a different employee, equipment, or test. That is an increase of 5% over 2020’s survey results. This year, 19% said they send results to another lab for verification and a second test, a decrease from last year’s 26%; 11% verify the procedure followed was correct, up from 7% previously; and 4% check for analyzer operational issues, same as last year.
Describing the steps taken to reduce the number of potential false positive test results, more people this year, 45%, verify all pre-analysis steps are performed correctly than last year’s 29%. Not as many, 20%, repeat the test with another method and compare results, compared to last year’s 29%; 17% repeat the test with the same sample and new extractions, not far off from last year’s 16%; and 15% do nothing, down from 21% last year. Others responded that they use a combination of these options.
Fernandes said that the best way to reduce errors is to automate testing processes. “Pre-analytical sample processing represents one of the most problematic areas in molecular laboratory testing,” explained Fernandes. “Highly skilled technicians perform repetitive manual tasks, like vortexing, decapping, and labeling tubes. These time-intensive, manual steps not only cause bottlenecks, but they increase the opportunity for human error and cross-contamination, with up to 75% of all laboratory error occurs during this phase. By automating these manual processes,1 labs have the potential to eliminate nearly 100% of human errors and could realize ~33% reduction in process steps, which allows highly-trained staff to accomplish more, focus on high medical value projects, and provide more balance to improve morale and job satisfaction.”
Donna McGowan, Marketing Manager of Indigo BioAutomation, pointed out, “No matter what instrument is in operation, the data release bottleneck is oftentimes the data. Although assays are built and validated on clean and well resolved peaks, sometimes the samples aren’t clean.” Algorithms and machine-learning have advanced accuracy, accelerating the release of results, managing sensitivity and specificity.
Molecular used in the lab
MLO also asked labs about non-PCR diagnostic technologies that they use in their molecular operations. Almost one-third, or 30%, said they use DNA and genetic testing, 22% use next generation sequencing (NGS), 12% use flow cytometry, and 6% of labs use liquid biopsies to screen for cancer genes with bodily fluids. More than half of labs either did not use the indicated technologies or used only PCR testing, while other labs mentioned using Sanger sequencing, as well as RNA analysis.
Purchasing mdx
“When planning to expand with new or more molecular assays, it’s important for labs to reach out to the assay vendor to make sure they can provide clinical evidence, educational and training material,” advised Nikos Pavlidis, the Vice President, General Manager of Molecular Diagnostics and Women’s Health & Cancer at Becton, Dickinson and Company (BD). “This will help you to better communicate to your customers, department and financial decision-makers the importance and value of the new assay, as well as make clear that your team will be fully prepared to collect samples appropriately and interpret the new results.”
Noting the challenges with supply chain, Pavlidis suggested, “Before making a choice, make sure that the assay/instrument manufacturer has control and will be able to provide the reagents, consumables, instrumentation and services required to maintain and support testing in your lab in a timely manner.”
He pointed to collection devices, pipetting tips or other consumables that the lab might need to source from a different supplier. “Having access to an all-inclusive consumable/assay source and a single, fully-automated molecular platform manufactured and serviced by the same vendor could prove to be a benefit in terms of both time and cost.”
The future of mdx
“Molecular testing was already on the rise prior to SARS-CoV2 and addressing the pandemic has pushed its growth and rapid adoption even further,” said Kim Futrell, MT(ASCP), MSHI, Senior Strategic Marketing Manager, Orchard Software. “Providing timely, highly accurate test results help providers gain insights to patients at the molecular level; that is exactly what we need as the next step toward improving outcomes.”
“Consumers have more awareness than before about molecular tests,” Ranalli said. “The average person talks PCR and molecular, knowing the differences between molecular, antigen and antibody testing.” She hopes the public awareness during the pandemic will inspire career choices in the younger generation.
“Working with inexperienced labs, helping labs grow to plan out the addition of molecular diagnostics in the lab, it was a collaborative effort that brought awareness to virology and better stewardship.” She predicted there may soon be a need for long-term COVID-19 testing, post-acute, especially for the immunocompromised.
Hart added that offerings of molecular diagnostics will continue to expand. “We can imagine a single analysis for the detection and confirmation of the common cold, influenza variants, COVID and even tuberculosis. We expect to continue to develop and expand protein assays towards the clinic at capillary and analytical flow rates as fully integrated workflows and even toward rapid analyzers. We expect also to see mass spectrometers that can be deployed closer to the patient. Finally, we truly see infectious disease identification and confirmation as a critical opportunity for the community as we move closer towards making the world a healthier, cleaner and safer place.”
Roche’s Fernandes said, “Healthcare was experiencing unprecedented change and disruption already, which has been further accelerated by the COVID-19 pandemic. What is expected of healthcare organizations is shifting from delivering services to achieving improved patient outcomes, while reducing costs.”
References:
- Hammerling JA. A Review of Medical Errors in Laboratory Diagnostics and Where We Are Today. Laboratory Medicine. 2012;43(2): 41–44.
About the Author
Marisa L. Williams
Editor
The author of more than 100 independently published books, Marisa L. Williams earned her Master’s at Johns Hopkins University, while interning on Capitol Hill, doing press and communications for the National Association of Community Health Centers. Creating her own Bachelor of Science degree at the University of Toledo, Williams blended pre-medical, pre-law, and laboratory studies, resulting in an interdisciplinary degree emphasizing Forensics. She has worked with Dr. Paulette Moulton (Levy), a dermatologist in Monroe, MI, as well as Dr. Elizabeth Triana, a family medicine practitioner specializing in hormone therapy, based out of Port Charlotte, FL; additionally, she has 20 years of experience as a multimedia journalist, is a third generation Realtor licensed in MI and FL, and has five years of college level teaching experience.