GM-CSF may be a key factor in controlling cytokine storm
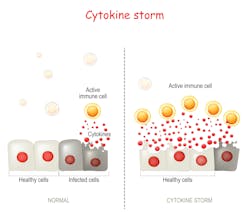
Cytokine release syndrome (CRS), also called cytokine storm or hypercytokinemia, is a potentially life-threatening condition that stems from the abnormal hyperstimulation of T-cells, resulting in the secretion of massive quantities of a variety of cytokines in the blood stream by T-cells, as well as most cells involved in the immune response, as they become engaged from the initial T-cell stimulation.
Despite the activation of the immune system through mechanisms similar to what triggers a normal response, the resulting cytokine and cellular proliferation that characterize CRS leads to an ineffective response, in particular, the inability to clear the infected/damaged cells at the origin of the hyperstimulation.
How cytokine storm develops
The development of a cytokine storm can result from an infection (Epstein-Barr virus, Cytomegalovirus, flu, COVID-19), an autoimmune disease (systemic lupus erythematosus, Kawasaki disease) and a number of other conditions, such as cancer and transplantation (graft-versus host disease). Another related condition is hemophagocytic lymphohistiocytosis (HLH), which includes familial, genetically driven forms triggered by defective cytolytic granule exocytosis, as well as secondary/reactive forms most often resulting from an acute infection. Meanwhile, macrophage activation syndrome (MAS), often assimilated to secondary HLH, is associated with infections, malignancies, and rheumatic diseases. Lastly, a cytokine release syndrome has also been shown to be associated with some newer T-cell engaging therapies, specifically those involving chimeric antigen receptor (CAR) T cells,1 of which it represents the most frequent severe adverse effect.
Laboratory abnormalities that are common in patients with CRS include cytopenias (all three lineages), elevated creatinine and liver enzymes, abnormal coagulation parameters (fibrinogen, D-Dimer), high CRP and high ferritin. However, a very large number of biomarkers apart from cytokines have been shown to be involved in the CRS response, involving markers of endothelial activation, such as Angiotensin 2 and Von Willebrand factor, and markers of vascular leakage, such as hypoalbuminemia and impaired lipid metabolism, with hypertriglyceridemia being one of the features of fHLH, to name just a few.
From a cytokine standpoint, IL-10, IL-6 and IFN gamma have long been the identified as being the core biomarkers of the CRS. Typically, IFN gamma is released by the stimulated T-cells or even tumor cells directly. Secreted IFN-γ induces activation of other immune cells, most importantly macrophages.1 The activated macrophages produce excessive amounts of additional cytokines, such as IL-6, TNF-α, and IL-10.
IL-6 plays a major role in the pathophysiology of CRS, since it has been correlated with major CRS symptoms, with a stepwise increase in levels with escalating severity, making its quantitative assessment valuable for patient management.
The double signalling pathway of IL-6, through membrane-bound as well as soluble IL-6 receptors, is thought to be key to the magnitude of the IL-6 effects. However, though targeted anti-IL-6 /anti-IL-6 R therapies have been attempted with some success in a number of CRS cases, including the ones linked to COVID-19, they didn’t permit CRS control in 100% of the cases, indicating that additional mechanisms need to be sought. Because monocyte and macrophages have been shown to play an active part in the development of the acute respiratory distress syndrome seen in CRS, as well as in the neurotoxicity following CRS in CAR-T cell therapies, researchers have scrutinized the role of GM-CSF.
The role of CM-CSF
In a recent study by Thwaites et al. of severe COVID 19 cases, extensive analysis of inflammation mediators was performed that identified GM-CSF as playing a distinctive role in the development of COVID-19.2 At the time of enrollment, IL-6, CXCL10 and GM-CSF, together with GDF-15 and CCL2, were the key biomarkers permitting patients’ classification into groups of increasing disease severity. By analyzing the levels of these biomarkers in the course of the disease progression, it was found that some (among which are IL-6 and GM-CSF) were stable over time and elevated since inception, making them good candidates for an early assessment of patients. Moreover, a comparison between fatal cases of influenza and COVID-19 cases, showed that even though IL-6 was elevated in all cases, GM-CSF (as well as IL-1 alpha) was specifically elevated in the COVID-19 cohort in contrast to the influenza cohort, supporting a prominent role of GM-CSF in the COVID-19 immunopathology.
Other neutrophil biomarkers, such as CXCL8, as well as neutrophil gelatinase associated lipocalin (LCN-2/NGAL), were also correlated with severity, confirming the interest in controlling neutrophilia and making GM-CSF an attractive therapeutic target.
Because monocytes and macrophages have been associated with the development of CRS and neurotoxicity following CAR-T cell therapies, attempts to neutralize the secretion of GM-CSF during these protocols have been investigated. Generation of GM-CSF deficient CAR T-cells, through CRISPR/Cas9 disruption of GM-CSF during CAR-T manufacturing,3 was proven not to affect the CAR-T proliferation or effectiveness while significantly reducing neuro-inflammation and CRS.
These converging discoveries confirm GM-CSF as a key contributor in the diagnosis as well as monitoring of CRS of various origins.
Limitations of IL-6 as diagnostic biomarker
Research panels have scanned dozens of biomarkers involved in the various pathways contributing to CRS severity in an attempt to identify a handful of biomarkers that could be universally applicable to the various CRS etiologies. However, at this stage, multiple independent discovery studies have led to as many different biomarker selections, showing that we are not ready for a one-size-fits-all solution.
In contrast, measuring IL-6 only as the main feature of CRS, is not sufficient to cover all cases with the granularity needed. Therefore, custom-made multiplex panels remain the tool of choice for CRS investigation, with the nature and number of markers dependent on the research goals. Being able to deliver an accurate, fully quantitative measurement of targeted biomarkers (at the very least IL-6, IFN gamma & GM-CSF) at bedside, within a 30-minute turn-around time, remains the goal of intensive care units monitoring severe CRS patients, even more so in periods of a pandemic where early triaging of patients is needed to optimize the healthcare services provided and minimize mortality. Careful selection of the epitope of choice/antibody pairs, use of standardized calibration materials when available, and confirmation of panel analytical robustness are essential for the reliability of these tests.
Such instruments and panels are currently brewing in many R&D departments; however, it will be some time, before they can be made commercially available, given the time needed for their approval and registration. In the meantime, making these tools available to clinical research remains the best way to improving patients’ prognosis, with the final IVD solution in mind.
References
- Shimabukuro-Vornhagen, Alexander et al. Cytokine release syndrome. Journal for immunotherapy of cancer. J Immunother Cancer. 2018; 6(56). doi:10.1186/s40425-018-0343-9.
- R. S. Thwaites et al. Inflammatory profiles across the spectrum of disease reveal a distinct role for GM-CSF in severe Covid-19. 2021. Sci. Immunol. Mar 10;6(57):eabg9873. doi: 10.1126/sciimmunol.abg9873.
- Sterner, Rosalie M et al. GM-CSF inhibition reduces cytokine release syndrome and neuroinflammation but enhances CAR-T cell function in xenografts. Blood vol. 133,7 (2019): 697-709. doi:10.1182/blood-2018-10-881722.
About the Author

Claire Hugeut, PhD
is Head of Biomarker Services at Randox Laboratories Ltd.