Effectiveness of NIV fecal immunochemical test for colorectal cancer screening
Colorectal cancer (CRC) is a leading cause of death among digestive diseases and the second leading cause of cancer-related death in the United States.1 According to the American Cancer Society (ACS), the lifetime risk of developing CRC is about one in 21 (4.7 percent) for men and one in 23 (4.4 percent) for women.2
Several health organizations have colorectal cancer screening recommendations, including the U.S. Multi-Society Task Force (MSTF), the U.S. Preventive Services Task Force and ACS. Colonoscopy is the most frequently used screening test in the United States, and it is often performed in average-risk, asymptomatic adults 50 years and older. However, only 60-65 percent of the eligible population is currently screened, a rate that is much lower than the goal of 80 percent by 2018.2 This alarming gap shows concerns over the test and strategy for screening.
Test methods for CRC detection
Many people experience anxiety and fear for a colonoscopy due to the need of full bowel preparation, sedation during the procedure and risk of bleeding, infection or bowel tears, which can often discourage people from having the screening done promptly—if at all. On the contrary, several other countries use annual or biennial stool blood tests or a combination of stool testing and lower endoscopy as the major screening strategy.3
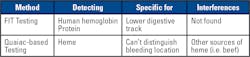
The stool blood tests detect “occult blood,” meaning blood that cannot be seen with the naked eye. Although stool occult blood may come from several possible causes, one important cause is the presence of polyps or cancers in the digestive tract. Two tests are designed to detect occult blood in stool. Each is designed to detect hemoglobin in red blood cells, but different components of the molecule.
The fecal immunochemical test (FIT)
FIT detects the globin protein of hemoglobin, specifically in humans. It does not detect globin from non-human blood, such as beef and other meats. Moreover, it can specifically identify bleeding from the lower digestive tract. (Hemoglobin from the upper digestive tract bleed would be broken down before it reaches the lower digestive tract.) FIT is considered more specific and sensitive than the guaiac-based test.
The guaiac-based test
Study: FIT versus colonoscopy
A recent study conducted by Indiana University School of Medicine and the Regenstrief Institute provides the strongest evidence to date to support recommendations that average-risk patients can safely opt for a non-invasive, easy-to-use FIT test instead of colonoscopy.4
The study reviewed and analyzed the findings from 31 colon cancer-screening studies with a total of 120,255 asymptomatic, average-risk adult participants who received FIT and a follow-up colonoscopy. “Average risk for developing colon cancer” is defined as having no family history of colon cancer, no personal history of inflammatory bowel disease, and no previous colon cancer or pre-cancerous polyps (for example, advanced adenomas).
FIT has demonstrated that for the average-risk adults, it had a moderate to high detection rate for colorectal cancer when compared to colonoscopy, the reference method. Sensitivity (true positive rate) in detecting colorectal cancer ranged from 71 percent to 91 percent, based on various thresholds of blood amount present in stool. It has an even higher specificity or true negative rate. FIT was able to rule out colorectal cancer 90-95 percent of the time when colonoscopies were negative. FIT sensitivity and specificity of detecting advanced adenomas in the colon or rectum were lower, but many of these benign polyps almost never advance into cancer.
The lead author, Dr. Imperiale, said, “Our analysis finds that FIT is a good ‘pre-screening’ test for average risk, asymptomatic adults, saving them hassle and the U.S. healthcare system costs.” Furthermore, “If annual FIT results remain negative, it buys you time until a colonoscopy may be required, and it could be the case that a colonoscopy for screening may never be necessary or required.”
This study warrants for more information to be shared with patients and healthcare practitioners about the use of annual FIT for colorectal cancer screening. It also demonstrates that FIT is not a “second-best” or less than “gold standard” strategy for average-risk individuals than colonoscopy. FIT might also be a better option for colorectal cancer screening in people under 50 years old, not only because colonoscopy is unlikely to be covered by insurance for this younger population, but also with the alarming 51 percent increase in colorectal cancer in this population since 1994.2
References:
- Peery AF, Dellon ES, Lund J, Crockett SD, McGowan CE, Bulsiewicz WJ. et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology 2012(143):1179-1187.
- Wolf AMD, Fontham ETH, Church TR, Flowers CR, Guerra CE, LaMonte SJ. et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin 2018(68):250-281.
- Navarro M, Nicolas A, Ferrandez A, Lanas A. Colorectal cancer population screening programs worldwide in 2016: an update. I 2017(23):3632-3642.
- Imperiale TF, Gruber RN, Stump TE, Emmett TW, Monahan PO. Performance Characteristics of Fecal Immunochemical Tests for Colorectal Cancer and Advanced Adenomatous Polyps: A Systematic Review and Meta-analysis. Ann Intern Med. 2019(170):319–329.
About the Author

(SB) Shi Bai, MD, PhD
is the chief resident of the Department of Pathology at University of Massachusetts (UMass) Memorial Medical Center. Her research focus is diagnosis of cancer and non-cancer diseases based on microscopic and biomedical examinations.

(SF) Shu-Ling Fan, PhD
is the director of clinical laboratories and point-of-care testing at UMass Memorial Medical Center, Worcester, MA. Her research focus is biomarker development for disease diagnosis. She is fully committed to providing quality results to support clinical diagnosis.