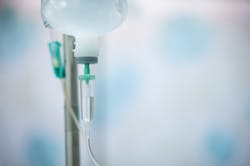
The U.S. Food and Drug Administration approved Hemgenix (etranacogene dezaparvovec), an adeno-associated virus vector-based gene therapy for the treatment of adults with hemophilia B (congenital Factor IX deficiency) who currently use Factor IX prophylaxis therapy, or have current or historical life-threatening hemorrhage, or have repeated, serious spontaneous bleeding episodes.
Hemgenix is a one-time gene therapy product given as a single dose by IV infusion. Hemgenix consists of a viral vector carrying a gene for clotting Factor IX. The gene is expressed in the liver to produce Factor IX protein, to increase blood levels of Factor IX and thereby limit bleeding episodes.
About the Author
Sign up for our eNewsletters
Get the latest news and updates