Quality is the mantra of medical laboratory professionals, but what is quality? What does it really mean? Why does it matter? Isn’t quality control (QC) good enough? Isn’t it the same thing as quality assurance (QA)? If we run quality control samples, why do we bother with external proficiency testing (EPT)? Isn’t plotting QC results on graphs good enough? What is the point of spending all this time and money on quality? Is it really worth it?
Quality is almost like a religion that everyone working in a medical laboratory is expected to believe in and practice. We run QC samples, controls, and the occasional EPT testing sample and may think that is good enough. Running these samples and controls is an excellent start but in itself is insufficient. Anomalous fridge/freezer temperature, QC, and EPT data need to be acted upon. There is no point in dutifully plotting out-of-range QC data or fridge and freezer temperatures without taking action. During accreditation inspections, I have seen beautifully plotted QC and temperature data that are outside their target ranges and yet no action had been taken to rectify the situation. Quality assurance is not just measuring and plotting numbers, it is about taking action to address any deviations from the target ranges, and whenever necessary, changing policies, procedures, and processes to reduce or eliminate errors and to try to reduce the probability of future errors occurring. This is the essence of continuous quality improvement (CQI) and of total quality management (TQM).
Quality control versus quality assurance
Quality control is a subset of quality assurance. They are not synonyms. Quality control is only the beginning of quality assurance. Quality assurance includes quality control; external proficiency testing; continuous and timely actions to address any deviations from target ranges; regular cleaning and maintenance of equipment; appropriately trained and qualified staff; a clean, safe, and sanitary physical workspace; correctly stored and processed reagents and samples; and the use of in-date reagents and equipment, including less obvious items such as personal protective equipment. The Clinical Laboratory Improvement Amendments (CLIA) of 1988 require a medical laboratory to have QC procedures to monitor the accuracy and precision of its testing processes. Laboratories may create an individualized quality control plan (IQCP) for their particular testing environment and patients.
How good is ‘good enough’?
If 99.99% of a laboratory’s reportable results are ok then that is good enough, right? If you think that 99.99% is good enough, then consider the following facts:
- 144 incorrect medical procedures would occur each day.
- 18 babies would be given to the wrong parents each day.
- 20,000 incorrect drug prescriptions would be written in the next 12 months.
- 567 pacemaker operations would be performed incorrectly this year.
- 810 commercial airline flights would crash every month.
Every day, two plane landings at Chicago O’Hare International Airport would be unsafe.
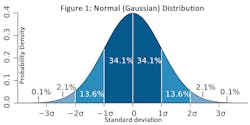
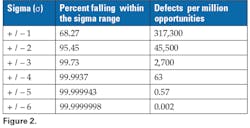
If 99.99% is not good enough, then what is? The answer is at least 99.9999998%. This is known as the Six Sigma approach to quality. ‘Sigma’ refers to the standard deviation of a set of data. (See Figure 1 and the Glossary of terms.) The goal of Six Sigma is to ensure that processes such as medical laboratory testing must not produce more than 3.4 defects per million ‘opportunities.’ A defect is any nonconformance to a standard requirement.You may be wondering why the Six Sigma goal is no more than 3.4 defects per million opportunities instead of no more than 0.002 (it is actually 0.00197 to be more precise) as seen in Figure 2. The answer is that in order to reflect real world experience, a Six Sigma process is allowed to move 1.5 σ on either side of the mean over time, where σ is the standard deviation (See Glossary of terms.) For example, if there is a shift of 1.5 σ to the right of the mean then 4.5 σ (6.0 - 1.5) is the acceptance area on the right and −7.5 σ ( -6.0 - 1.5) on the left. With this shift, the rate will be 3.4 defects per million opportunities: if P = the probability density then,
P(z= -7.5) + P(z >+4.5) = 3.4 x 10-6 = 3.4 defects per million opportunities, where ‘z’ is the number of standard deviations from the mean (the z-score).The true costs of quality
The total cost of quality is not always obvious. Some costs are highly visible but others are hidden or ‘invisible’ without looking deeper (See Figure 3: The Quality Iceberg). The total cost of quality can be measured. Quality-related costs can be divided into four categories:
1. Costs associated with preventing errors (prevention costs).
2. Costs associated with monitoring quality (monitoring costs).
3. Costs associated with internal laboratory failures.
4. Costs associated with pre- and post-analytical errors.1. Prevention costs
- The cost of a dedicated quality assurance (QA) person.
- A portion of the time dedicated to QA by the laboratory director, managers, and other staff.
- Preventive maintenance contracts etc.
- A portion of laboratory information system (LIS) costs, office supplies, printing, copying, etc. dedicated to QA.
- Competency assessments of staff, continuing education, and other training.
2. Monitoring costs
- Quality control (QC).
- Calibration and control reagents and analyses.
- Temperature monitoring and alarm systems.
- Annual accreditation, including inspection costs and annual accreditation fees.
- Self-inspections required by an external accreditation agency.
- External proficiency testing.
3. Internal laboratory failure costs
- Expired reagents and disposables.
- Repeated QC and calibration testing.
- Correction of transcription errors, specimen processing, and accessioning errors.
- Repeated sample testing. Failure to follow SOPs (standard operating procedures) and cost of retraining staff. For example, correction of testing procedure errors, performing the wrong test on the wrong sample, etc.
4. Pre-and post-analytical costs
Goodwill
Medical laboratory results must be timely and trustworthy. Physicians and other healthcare professionals need to know that any test results that they receive from your medical laboratory are trustworthy. Trustworthy results are your medical laboratory’s raison d’etre, i.e., they are why your lab exists and why you have a job. Physicians and other healthcare professionals rely on these tests for the diagnosis and treatment of patients and also to monitor therapeutic drug levels, among other things. Quality is your responsibility whether it is in your job title or not. Successful medical laboratories know that their reputation is paramount. It takes time, money, and total quality management to build a great reputation. On the other hand, a good reputation can be lost in seconds if the quality of your products and services is poor or inconsistent.
Consistently high-quality builds goodwill. Goodwill is an intangible but valuable asset. It is the value of a company’s reputation. For example, Microsoft, Amazon, and Apple all have very large intangible assets (goodwill) built on their reputations. A company’s intangible (goodwill) value may far exceed the value of its tangible assets such as buildings, inventory, land, etc. In business, including medical laboratories, reputation is everything. Reputations must be built and maintained. It is already too late if you only begin to think about quality just before the next external accreditation inspection is due. Think, live, and breathe it every day. Quality means doing it right even when there is no one looking. Quality is everyone’s responsibility. Quality is not just a theory or a mantra: it is a lifelong habit!References
1. Homepage. College of American Pathologists. Published November 2, 2017. Accessed September 27, 2023. https://www.cap.org/.
2. Muenz R. The main goal of Six Sigma implementation. Today’s Clinical Lab. Published March 5, 2020. Accessed September 27, 2023. https://www.clinicallab.com/trends/the-automated-lab/the-main-goal-of-six-sigma-implementation-21937.
3. Pant V, Pradhan S, Gautam K. Basics of laboratory statistics. EJIFCC. 2023;10;34(2):90-102.
4. Westgard JO, FACB. “Westgard Rules” and multirules - Westgard. Westgard.com. Accessed September 27, 2023. https://www.westgard.com/mltirule.htm.
About the Author

Ian Wilkinson PhD, DClinChem, CSCC(Cert), MBA
is the author of numerous books and articles on management, business, clinical biochemistry, the history of medicine, and humor. His latest book, The Hitchhiker’s Guide to Business & Management: An Aaaaaarrrrrrgggggh to Zzzz is available as an e-book and in paperback format at: https://www.amazon.com/Hitchhikers-Guide-Business-Management-Aaaaaggggghhhhh/dp/B0CFZJK6JJ/ref=tmm_pap_swatch_0?_encoding=UTF8&qid=1692371115&sr=1-1.