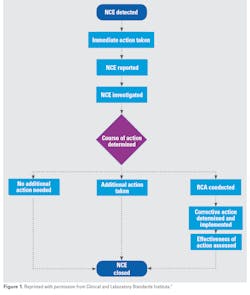
A nonconforming event program reduces misidentification errors
Researchers estimate that more than 160,000 adverse patient events occur each year in the United States as a result of errors in identifying patient specimens used in laboratory testing.1
Regulatory and accreditation organizations require policies, processes, and procedures to ensure correct patient and specimen identification, but errors occur frequently. The potential for significant harm exists from test results reported on the wrong patient, not only to misidentified patients, but also to patients whose healthcare decisions are based upon test results from misidentified specimens. The risk of harm in both scenarios is high, and laboratories must establish stringent policies for identification errors. Of all pre-examination processes, improperly identifying patients and incorrectly labeling diagnostic specimens have the most potential to result in catastrophic consequences.2 Potential harm to the patient from the lack of standardization in identification procedures in healthcare include misdiagnosis, incorrect treatment, failure to treat an existing condition, unnecessary surgery, injury, disability, and death.3,1
Patient and specimen misidentification errors
Misidentification can present in the laboratory in a variety of ways, including:2
- A specimen is received unlabeled.
- A specimen is incompletely labeled.
- An examination request is received with incomplete or incorrect patient ID information.
- The patient ID information from the specimen and examination requests do not match.
- The specimen and examination request match, but the specimen was collected from the wrong patient.
The patient identification process
Accurate patient identification is necessary at all points where the patient interacts with the healthcare system. While healthcare professionals recognize the full name and date of birth as two possible identifiers, neither name nor date of birth alone can identify a single unique patient.
Most healthcare systems have created a code, the patient-specific identifier, which is used to link the uniquely identifiable patient to his or her medical record. Confirmation of the patient’s identity using photo identification or another acceptable demographic indicator is also recommended.2 In addition, all inpatients must have ID bands affixed to their person, unless exempted by facility policy (eg, residents in long-term care facilities, patients in psychiatric wards). If any discrepancies are identified, specimens should not be collected, until all inconsistencies are resolved. Any discrepancy, however minor, must be reported to the responsible caregiver as determined by facility policy.5
Healthcare professionals must ensure that the specimen is collected from the individual designated on the examination request and/or labels. Examples of high-risk situations that put patients at risk of being misidentified include: 2
- Siblings or twins
- Newborns
- Common names
- Look-alike or sound-alike names
- Multiple patients in the same room
The five main elements of every patient specimen label include: (1) patient name, (2) a unique patient identifier, (3) date of birth, (4) specimen collection time and date, and (5) a designated space for the collector’s identification (initials, signature, code).4 Specimen collection containers must be labeled immediately after collection, in the patient’s presence, by the healthcare professional who collected the specimen, unless the specimen is self-collected. When circumstances result in a specimen being labeled by someone other than the healthcare professional, the person labeling the specimen must witness the collection and that the patient was identified properly.2
Patients must confirm to the best of their knowledge that the identifiers on the labeled specimen(s) are correct. For patients without ID bands who are unable to participate in the confirmation process, specimen identifiers must be confirmed by a caregiver, relative, or friend. After labeling, all information, including specimen site, date, and/or time of collection (if relevant), must be verified to match exactly while still in the presence of the patient.
It is the responsibility of the healthcare professional to compare the information: 2
• As verbally stated by the patient when initially identified
• On the facility ID band (if required to be worn by the patient)
• On each labeled specimen
• On the examination request if present at the time of collection
Any unused labels or labeled collection containers must be appropriately discarded to prevent their accidental use with specimens belonging to other patients.
It is important that laboratories have policies and procedures in place regarding the handling of specimen containers that arrive without labels, or when there is a mismatch between the labeled specimen container and accompanying paper requisition slip or other documentation.4
About the Author

Joanne P. Christopher, MA, ELS
is a content specialist for the Clinical and Laboratory Standards Institute (CLSI). She is a board-certified life sciences editor and a member of the American Medical Writers Association (AMWA). Joanne has been writing for the healthcare industry for 15 years.