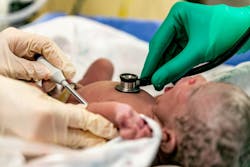
Magnolia Medical Technologies announced the commercial availability of its new Steripath Micro Initial Specimen Diversion Device, which is designed for children’s hospitals.
Use of the new Steripath Micro device in two leading children’s hospitals has shown a zero percent contamination rate over the last three months, the company contends, saying that false positive results, which occur as a result of specimen contamination, are common.
Steripath Micro is a small, lightweight device designed to standardize the blood culture collection process and reduce blood culture contamination. The device has received 510(k)-clearance from the U.S. Food and Drug Administration.
About the Author
Sign up for our eNewsletters
Get the latest news and updates