Quality control for SARS-CoV-2 testing
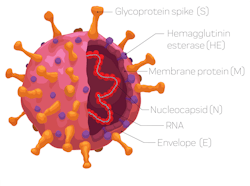
To identify, treat, and contain SARS-CoV-2, the virus that causes COVID-19, clinicians worldwide rely on timely and accurate diagnoses. Because of the fast-paced development of test kits, it is important that laboratories maintain vigorous quality control (QC) practices to establish confidence in the reliability of test results. This article outlines some suggested best practices for good quality control when testing for SARS-CoV-2. Specific attention is given to practical approaches for utilizing quality control protocols for different testing methodologies.
Testing methodologies
There are currently three different test methodologies in practice to identify whether a patient has been exposed to the virus. Each methodology is either based on detecting the viral RNA or viral proteins (antigens) directly, or detecting the patient’s antibodies, which are an immunological response to being exposed to the virus.
- Molecular tests are designed to detect the presence of viral RNA in a nasal or nasopharyngeal swab or wash/aspirate.
- Antigen tests are designed to detect the presence of viral proteins in a nasal or nasopharyngeal swab or wash/aspirate.
- Serology or antibody tests are designed to detect the presence of human immunoglobulins in blood.
Molecular testing
Molecular testing mainly relies on the analysis of nasal/nasopharyngeal or wash/aspirate samples by PCR methods. The identification of the virus is based upon detecting specific genes. Molecular tests use one or more of these genes to identify the virus.
When selecting a quality control material for your molecular test, you should ensure that the multiple gene targets used in the assay are also available in the control material. Reacting to testing supply shortages, some labs are validating and running multiple molecular assays so that if one system is down due to lack of supplies, testing can continue on another system. In these scenarios, inventory management can be streamlined by using a control containing all the gene targets covered by the laboratory’s different molecular assays.
Commercially available control materials fit into three categories:
- Synthetic RNA: These materials, with one or more target genes, are designed by formulating synthetic RNA transcripts, which contain the unique genes. They are non-infectious and can be quantified at low positive concentrations.
- Inactivated or live virus: Viral particles can be chemically or heat-inactivated to be rendered non-infectious. Whole genome controls fit into this category.
- Plasmid DNA: Purified /intact bacterial cells containing a synthetic SARS-CoV-2 sequence. These also contain one or more target genes.
An important step in the testing process is the extraction or purification of the viral RNA from the biological sample. Patient samples are mixed with a lysing buffer to release the genetic material. The RNA is then separated from DNA and other proteins in the sample with specific extraction reagents. To test this extraction process, quality controls should contain human genetic material or a protein coat similar to the actual virus (armored control). A negative control material should contain human genomic DNA, which mimics the actual patient samples and allows laboratories to validate performance of the entire molecular assay process – including extraction, amplification, and detection.
Most control or reference materials are quantitative and have a concentration provided in the product insert. A well-characterized quantitative material can also be used for various aspects of the validation or verification process, such as establishing the limit of detection (the lowest analyte concentration that the test method can detect).
As with other test-method quality-control procedures, you should use independent, third-party quality control materials. Not only are these advantageous for efficiency by being able to be used across multiple platforms, they also offer an unbiased, independent performance check.
QC practices
To assist in the development of pragmatic operational QC practices, there are a number of resources offering guidance.
For example, in terms of frequency, the WHO recommends that QC testing be performed with each run and has noted that, “each nucleic acid amplification test (NAAT) run should include both external and internal controls and laboratories are encouraged to participate in external quality assessment schemes when they become available.”1
The College of American Pathologists (CAP) Microbiology checklist (MIC 65200) states that for (Daily QC - Molecular-based Testing), “molecular-based quantitative and qualitative tests, controls are run at least daily, or more frequently if specified in manufacturer’s instructions, laboratory procedure, or the CAP Checklist, and when changes occur that may impact patient results.”2
Clinical Laboratory Improvement Amendments (CLIA) has provided additional QC guidance for Emergency Use Authorization (EUA) COVID-19 testing, which in certain conditions allows for reduced QC without requiring an IQCP. CAP, Joint Commission, and other accrediting bodies have followed suit. However, given the sensitivity around this testing, reduced QC should not be taken lightly. For optimal results, however, it is recommended that labs adhere to the following CAP guidelines for QC of molecular testing:
- Quantitative tests: three controls at least daily, including a negative control, a low-positive control, and a high-positive control.
- Qualitative tests: daily positive and negative controls.2
While most of these molecular methods are reported qualitatively, many produce an underlying quantitative result as a Cycle Quantification (Cq) or Cycle Threshold (Ct), identifying the cycle number at which the sample’s reaction curve intersects with the threshold line. That value is then translated into a qualitative result for purposes of test reporting. The resulting value can then be evaluated and used as a performance indicator for the test. Other quantitative methods can also produce additional quantitative values (ex: copies/uL).
When quantitative results are generated, laboratories can establish their own target means and ranges. Although the product inserts may provide target values, good QC practice dictates that the lab establish its own targets. This can easily be performed by running the control at least ten times over different days or runs.3
When setting an acceptable QC range, some labs have their own ranges defined based on experience or clinical use (for example +/- 3 Ct). However, as with traditional quantitative QC procedures, the lab can use an existing standard deviation (SD) or coefficient of variation (CV) from a previous control lot, or establish a new SD or CV by initially measuring 20 control results and then updating these estimates when more results are available.
Documenting the results in Levey-Jennings charts can help to identify trends and possible shifts in the test method performance. Further confidence is gained by participating in a peer-comparison program and proficiency testing program. These tools provide insights, not only for the lab itself, but also as to how a test method or reagent log is performing across many labs.
Antigen testing
At the time of writing this article, there was only one test approved by the U.S. Food and Drug Administration (FDA). It is a cartridge-type rapid test utilizing a sandwich immunofluorescence technology to identify the SARS CoV-2 antigen.
As with many point-of-care (POC), lateral flow tests, this test provides a built-in procedural control, but also provides a positive and negative external quality control.
The quality control minimal requirements for point-of-care tests are often specified like this:
- Once for each new lot, or new shipment of kits, provided that each different lot received in the shipment is tested.
- In accordance with local, state, and federal regulations or accreditation requirements
Antibody testing
According to interim guidelines from the Centers for Disease Control and Prevention (CDC), there is currently no identified advantage of assays whether they test for IgG, IgM and IgA or total antibody.4 Tests that detect binding antibodies can be classified via two broad categories:
Laboratory tests that use ELISA (Enzyme-Linked Immunosorbent Assay) or CIA (Chemiluminescent Immunoassay) methods for antibody detection. Based on the reagents, IgG, IgM, and IgA can be detected separately or combined as a total antibody analysis.
Rapid point-of-care tests generally are lateral flow devices that detect IgG; IgG and IgM; or total antibody presence in the sample.
QC practices
Depending on the methodology used and the types of antibody detected, different control materials are available. When running multiple assays in a lab, an advantage of a total antibody control (IgG, IgM and/or IgA) is that it can be used for both total antibody tests as well as specific antibody tests, which simplifies inventory management issues. As expected, individual antibody controls can only be used for the specific, designated antibody test.
Some kits might include their own control material (for example ELISA methods) that are required to verify that tests are performed according to procedures and/or are used to derive assay cutoff value. As ISO 15189:2012(E) sub-clause 5.6.2.2 references, “Use of independent third-party control materials should be considered, either instead of, or in addition to, any control materials supplied by the reagent or instrument manufacturer. The laboratory shall use quality control materials that react to the examining system in a manner as close as possible to patient samples.”5
According to CLSI-C24-A4, “advantages for participating to an interlaboratory QC program are: Verifying that a laboratory is producing QC results that are consistent with other laboratories using the same measurement procedure, and thus demonstrating that the laboratory is using the measurement procedure correctly.”3
Another aspect to take into consideration for both molecular and serology testing is the QC validation linked to “batch testing, where a direct correlation exists between the quality of a control material examination and the patient specimen examinations in the batch. With discreet testing, where each specimen is examined individually, there is no longer a direct correlation between the quality of a control specimen and the quality of a subsequent patient specimen.”6 Bracketing patient samples with QC specimens is one of the most-used solutions for discrete testing platforms.
Conclusion
With the current climate of urgency and the emerging new tests and methods being approved through emergency use authorizations from the FDA, it is important to vigilantly control the performance of diagnostic and surveillance tests. With faster development and production, logic dictates that QC protocols should be bolstered to ensure performance issues are quickly identified and resolved. Standard good quality-control practices for molecular and serology methods certainly apply for the new SARS-CoV-2 tests.
When using different testing methodologies in the laboratory, selecting a quality control product that can work for multiple assays can be beneficial. Selecting independent third-party quality control materials with a matrix as close as possible to the sample material will also support a solid quality control design. As SARS-CoV-2 assays continue to evolve, so should quality control practices be strengthened to provide the best possible testing outcomes for the public during – and beyond – this pandemic.
References:
- Laboratory testing for coronavirus disease 2019 (COVID-19) in suspected human cases. World Health Organization. WHO.int. https://apps.who.int/iris/bitstream/handle/10665/331329/WHO-COVID-19-laboratory-2020.4-eng.pdf. 2 March 2020. Accessed 1 June 2020.
- Microbiology Checklist MIC 65200. College of American Pathologists. CAP.org. https://elss.cap.org/elss/ShowProperty?nodePath=/UCMCON/Contribution%20Folders/DctmContent/education/OnlineCourseContent/2017/LAP-TLTM/checklists/cl-mic.pdf. 21 August 2017. Accessed 1 June 2020.
- Parvin, Curtis A. Statistical Quality Control for Quantitative Measurement Procedures: Principles and Definitions, 4th Edition. CLSI. 2020; C24 A4.
- Interim Guidelines for COVID-19 Antibody Testing in Clinical and Public Health Settings. Center for Disease Control and Prevention. 23 May 2020. Accessed 1 June 2020.
- Medical laboratories – Requirements for quality and competence. International Organization for Standardization. ISO.org. https://www.iso.org/obp/ui/#iso:std:56115:en. 2012. Accessed 1 June 2020.
- Yundt-Pacheco J, Parvin CA. Validating the performance of QC procedures. Clin Lab Med. 2013;33(1):75‐88. doi:10.1016/ j.cll.2012.11.006
About the Author

Peter Deman, PhD, MBA
serves as International Scientific and Professional Affairs Manager for Bio-Rad Laboratories Quality Systems Division. He works with clinical laboratories and societies around the world in developing, educating and promoting quality control concepts, strategies and best practices and is a member of the EFLM Working Group for Preanalytical Phase.

Nico Vandepoele, BSc
serves as Scientific and Professional Affairs Manager for Bio-Rad Laboratories Quality Systems Division. He works to promote an understanding of laboratory regulations and best practices as they pertain to QC and EQA/PT programs.