Most medical technologists and technicians, responsible for outputting test results as quickly and reliably as possible, hate it when their smooth flow of work is abruptly interrupted by an out-of-control QC rule flag. Suddenly they are faced with delayed reports, the prospect of a complicated technical investigation, and a litany of questions they must ask themselves, such as:
- “Is the out-of-control condition real?”
- “Do I really have a problem with the analytical system?”
- “If I do have a problem, when did it start and how many patient samples are affected?”
- “What should I do first?”
- “What should I do next?”
The laboratory would have much less of a conundrum if it were using a new QC rule proposed in a 2012 Clinical Chemistry article.1 This rule allows, as part of its process control, the possibility of a second set of QC measurements when the first results are inconclusive.
The rule is called a Repeat 1:2s QC Rule, where 1:2s designates that the “run” is rejected when a single control is out 2 standard deviations (2s). This helps improve the rule’s ability to verify whether the process is out of control. If the rule triggers a second set of QC results and the repeat results are within their 2 standard deviation (SD) limits, then the laboratory can assume the condition was a random occurrence and patient results can be reported. However, if any repeated QC result is outside its 2 SD limits (regardless of the control material) the run is rejected and patient results are held for retest.
The test system and control system
Before troubleshooting begins in earnest, the laboratory should consider the possibility that common failure modes of the test system or the control system may be the cause of the out-of-control condition.
The test system includes the reagents, hardware, and software. It may require more in-depth investigation and analysis, but the laboratory should at least first consider those few things in the test system that are known to cause problems or have a history in the lab of causing problems. These could include such things as the reagent integrity, the tubing and pumps used for sample and dispense, and the light source.
The control system includes the control materials, the mean and standard deviation used to set the Levey-Jennings chart, and the process control rules applied. Any of these could be the cause of the out-of-control condition. The technologist or technician should verify the control materials are in date, have been properly stored, and have been properly prepared. Attention should be given to open vial stabilities published in the product insert. Most control materials must be kept between 2-8oC, even after reconstitution, so if the control material is kept at room temperature on the analytical bench most of the day, open vial stabilities and consequently control results may be compromised.
The mean and standard deviation are key to an effective Levey-Jennings chart. Both must be calculated using sufficient data collected over a period of time, allowing for multiple calibrations, multiple reagent lots, maintenance, and multiple operators when technique may be an issue. If either the mean or standard deviation is out of synchrony with actual test performance, the Levey-Jennings chart will frequently issue out-of-control alerts.
Recalibration is often the first but misguided reaction to an out-of-control condition. Keep in mind that every time the laboratory calibrates, it creates an opportunity for introduction of new or additional measurement error. Consequently, if a laboratory calibrates more frequently than recommended by the manufacturer, it is one indicator that the quality control system is likely out of synchrony with current test performance.
Random error and systematic error
If the quality control system is found to be appropriate and effective, then troubleshooting begins. First, the type and approximate size of the error must be characterized, because this will lead the investigator to possible specific causes which are either random or systematic. This can be accomplished using control data from previous QC events (recent and historical QC), or the laboratory may choose to take a more contemporary approach by testing multiples of additional controls at different concentrations (Ns of 2 to 8 or more) and then examining the data for clues as to the magnitude and cause of the out-of-control condition.
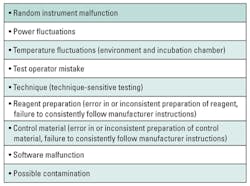
If the control pattern suggests random error, then the laboratory should investigate common contributors to random error (Table 1). If a cause can be found, corrective action is taken. If a cause cannot be found, then the laboratory should perform comprehensive instrument maintenance followed by recalibration. The control materials are retested, and if the results are out of control, then the laboratory must continue to sequester all patient results and undertake a root cause analysis. If the control results are in control, then all patient samples are retested and reported. Remember, random error conditions occur, well, randomly, so all patient results prior to the correction are suspect.
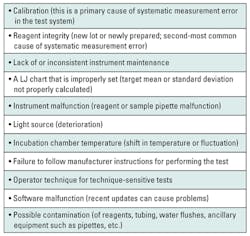
If the control data pattern suggests systematic error, then the laboratory should investigate common contributors to systematic error (Table 2). Systematic measurement error is much easier to find and identify than random error. The difficulty is in determining at what point in time the systematic measurement error occurred, making the number of patient samples between QC testing events really important. For example, if a laboratory only tests quality control materials every 500 patient samples, then it faces the difficult task of deciding which and how many of the previously tested samples need to be retested. In some cases, time may be a factor as well. It is possible that the lapse in time for 500 samples may exceed the analyte stability, invalidating any chance to retest. On the other hand, a laboratory that tests a QC material every 50 patient samples is in a much better position to correct any bad patient results.
Once the cause for the systematic measurement error is found and corrected, the test is recalibrated. Quality control materials are retested. If the results are out of control, then the laboratory must sequester all test results and undertake root cause analysis. If the results are in control, then all patient samples that are believed to have been affected by the out of control condition are retested and reported.
REFERENCES
- Parvin C, Kuchipudi L, Yundt-Pacheco J: Should I repeat my 1:2s QC rejection? Clin Chem, 2012;58(5):925-929.
W. Greg Cooper, CLS, CQA, MHA, is a licensed clinical lab scientist in the state of California. He is an ISO 15189 auditor and has a consulting business focused on activities that improve laboratory quality.