Overcoming data management challenges: The biggest challenge of all is….us
New or newly engineered analytical technology has brought about an array of analytical systems for laboratory testing just as information technology has brought faster and more innovative ways of displaying the information for clinicians. All are great advances for facilitating patient care, but this author’s experience over the decades has shown that to make it all work together most effectively, providers, laboratorians, and information technology professionals, both institutional and external, must work as an integral unit with a mutual professional respect for each other’s operational needs and perspectives.
A recent article in the October issue of MLO by J.S. Tony1 really piqued this author’s interest, since over a long professional career, managing information/data has too frequently emerged as an issue that surpassed many technical and operational challenges.2-6 However, the writer began to reflect on its apparent simplicity as being the panacea for the ills of communicating critical information from a wide range of laboratory sources. Not taking away from the concept of an all-in-one LIMS as described by the article’s author, which he stated clearly and with appropriate enthusiasm — an enthusiasm I share although expanding the scope to meet laboratory and provider needs more fully.
Despite this ‘operational’ enthusiasm, attention should be drawn to some serious concerns about implementation and enthusiastic acceptance by the end user of any such system. This concern is based on issues related to getting the correct information to the end-user/provider in a rapid and unambiguous manner appropriate to the need.7
Salient points: Critical and point-of-care patients
Many analyses are performed not only in the central laboratory but in POC systems that are controlled by the lab and in systems that are internal but independent of the central lab. All data/information should go to the patient record, which gives rise to inevitable comparisons and potential for confusion especially when analysis is with different instrumentation and report nomenclature. Consequently, we will use some examples from POC testing. While POC testing may be the best example of the needs as concluded here, other areas of lab testing and reporting undoubtedly have analogous issues.
The usual issues of integrating information from disparate sources is even more significant in POC and critical care testing since immediate critical decisions may be made, and the accuracy of communication of both quantitative results and their specific meaning is crucial. Optimally that meaning must include the nature, source, and collection time of the specimen as well as the quantitative and qualitative characteristics of what is being reported. The information must be understandable to and among the various professional providers — not just the scientists who developed the measurements, protocols, and the naming/symbols used. To bring this about, our experience shows that significant pre-work is necessary, especially including the information technology (IT) professionals who develop and maintain both analytical and reporting systems. I could also cite examples of reports and formats we thought would be ‘great’ but when put into practice were totally rejected by medical staff, because we did not consult with them on their needs or wants.
While some of these concerns are obvious and primarily technical issues with solutions already made available by the various technical or professional organizations, the real integration of systems goes beyond the technical. We believe that dialog between central laboratory and IT professionals is only the beginning. Direct providers and central laboratory as well as ‘independent’ POC testing areas must all be part of the design and implementation of the final output of any such system, including the inevitable compromises. This may be a challenge, since each group may speak a different professional/technical dialect, if not at times a different language, and quite possibly have a different view of intrapersonal communication on the clinical and analytical aspects. Based on this writer’s experience, successful implementation of useful information reporting systems is less likely or will take longer if these different dialects and communication styles are not acknowledged and accommodations made by both the direct participants and their management. The associated issues are not resolved by one or two mega-meetings, each group identifying their requirements, IT implementation, and then voila-success! Success only results from a lot of arduous work (sometimes boring and repetitive but still challenging). Repeated explanations and discussions within small and large groups and reflection in between are all part of an effective process. Once believed complete, one finds that it is not complete: test/evaluate the results, make corrections, and then try again, putting it into effect one related piece at a time.
Attempting any such program requires a real and long-term commitment on the part of not only those clinical and technical specialists but more importantly of their direct and high-level management. The developer/supplier of an ‘all-in-one’ LIMS must recognize this and then facilitate the process as needed.
Communication and complete ID of results exemplified in POC
An example shared from POC testing and reporting results may help understand the generalities stated above. (And we would expect that other specialty areas may have other similar specimen and reporting that might be added to this.) A specific example of a situation in which analytical/clinical ambiguity may exist, and consequently impact patient care, is that of the enhanced blood gas analyzer (eBGA-i.e. the blood gas systems that also measure electrolytes, CO-oximetry, glucose, etc.) or any similar multi-measurand system. The software/firmware is already in place and data/information available to the provider. Since clinical action may take place before the full system reports are available, subsequent confusion will be reduced if the reports from different devices are similar in appearance and detail.
Basic identification: 1) Identifying patient and specimen type/source and collection time/date clearly. Recall that a ‘blood gas,’ or an ‘enhanced blood gas’ is done on blood, but the blood can be from various sources — arterial, capillary, venous, etc., and each with different clinical meaning. 2) Identifying the measurand’s entity/quantity type (i.e., analyte or parameter). For example, carbon dioxide tension in venous whole blood. -pCO2(vB), mmHg/kPa. [Kind of quantity ‘p’ (tension); measurand identity –‘CO2;’ specimen source –‘v,’ venous; and specimen type – ‘B’ (blood).] All International Federation for Clinical Chemistry (IFCC) / International Union of Pure and Applied Chemistry (IUPAC) symbols.
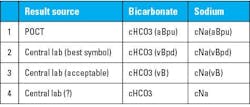
Technology effect identification: Specific technologies impact reported results. (Are all tests performed on ‘whole blood’ in fact, concentrations in whole blood? No, they are not!) What does a sodium concentration mean? Is the central lab value different from the eBGA value? The symbol set for molar concentration of sodium from an eBGA should be cNa(aBpu). [‘c’ molar concentration; Na’–sodium; ‘a’– arterial; ’B’-Blood; ’pu’ – plasma, undiluted.] The symbols are all IFCC/IUPAC, but with the subscripted ‘u’ referring to the Clinical and Laboratory Standards Institute (CLSI) / National Institute of Standards and Technology (NIST) calibration protocol. Similarly, Is a ‘bicarbonate’ the same thing as a ‘plasma bicarbonate’? The symbol set for bicarbonate from an eBGA should be cHCO3(aBpu).
With the specific identifying symbols as a part of the name, there is no question, in each case is a concentration (molar) of the ion from an arterial blood specimen reported from undiluted plasma. The central lab would ideally report for example cNa(vBpd), with collection time included in an unambiguous report. Use of these established symbols reduces the size of the name on the report while providing the most complete information to the provider.
External sequelae: Some laboratory system/POC reports may be used in other (non-lab) computations). Consequently, expect this, and label results as part of the name accordingly. Thus, as one example, a ’mixed’ venous blood gas would report -cO2(vmBt) representing the concentration of oxygen in the mixed venous whole blood specimen as a total of the dissolved and that bound to hemoglobin — clear and unambiguous both analytically and clinically.
Thus, clear, timely, and unambiguous reporting to the clinical provider of direct patient care involves a complex interaction among users and providers within the institution and within the relevant parts of the institution as well as with the manufacturers of the analytical systems and LIMS vendors. Many, if not most, of the technical solutions are already available to central laboratories, especially through the International Federation for Clinical Chemistry (IFCC) for complete and unambiguous nomenclature and symbols, and by CLSI and NIST for standards (material and paper).8,9
Symbols as names on patient record/information management display: The question might be, are these technical solutions widely known and applied by laboratory staff, to say nothing of the medical professionals who use the information? An example of existing symbols as prescribed by IFCC/IUPAC or proposed to them13 is shown in the table below. These symbols also include designations that identify specimen treatment effects by the analysis according to the CLSI/NIST guidelines, thus making the significance of values immediately obvious (See Table 1).
Other regulatory requirements for having certain information available on the laboratory report make sense, but is the information where it is really needed, or does the provider have to hunt for it? Does the hunt for essential information frustrate the provider to the extent that they contact the lab for information, thus wasting both provider and lab staff time?
Other specimen characteristics may also need to be identified for clearer documentation and for both clinical and analytical use. Consider: 1) ‘arterialized’ capillary specimens as collected, are different from both arterial specimen and untreated capillary specimens; 2) venous or mixed venous specimens are also different and may be used differently. Finally, POC systems, (standalone and multicomponent), measure and report other quantities on an undiluted specimen in the plasma fraction of the whole blood-potentially affecting result value. These and other collection and measurement-related conditions can affect results and their interpretation.
Reporting issues have been exacerbated as more testing has been done using ‘enhanced’ blood gas analyzers’ (eBGA’s) since many of these systems also have been simplified to the extent that professional staff operating them are not as familiar with the symbology that can be used or the significance of the technology on clinical practice.
Not only is it important to label specimens as to type/nature and source, but in reporting and setting requirements for instrumentation and reporting/charting system used. Those responsible for measuring, as well as integrating the measurement devices output with system wide reporting must make provisions for complete specimen-result characterization. Testing experts may need to get together with IT and other clinical experts to assure that what is reported is identified/reported unambiguously.
A more encompassing solution
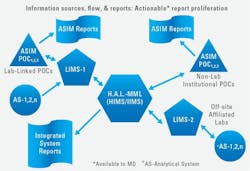
To accomplish the overall goal of better and more efficiently provided information to providers, it is essential to have a developed rapport among the medical and measuring professionals by, for example, constructively participating in each other’s staff meetings/professional committees,8,9 as well as the more traditional in-service training. With the central lab taking the lead in organizing the discussions and using the symbols and protocols already available through IFCC13/IUPAC, CLSI, and NIST, the output of various information management systems can be enhanced and operational management clearly improved. See Figure 1.
For the many institutions now using both traditional measuring methods and eBGA’s, accurate and complete specimen measurement differentiation will enable the diagnostic/therapeutic/operational advantage of the various technologies that are now analytically fully integrated. This ensures proper comparison of numeric values when viewed on patient’s charts, which may have the same measurand reported using other non-direct systems — making more work for the IT professionals in set-up — but important for both analytical accuracy and clinical application.
The mantra might be to conceive, design, adopt, adapt, integrate, educate, and communicate to enhance improved patient care for these most critical of tests performed in point(s) of care. That involves not just a great all-in-one LIMS, but one fully integrated with the HIMS/LIMS and designed/put together by a total and collegial healthcare team.10,11
References
1. Tony JS. Overcoming data management challenges with an all-in-one LIMS. Medical Laboratory Observer. Published September 21, 2023. Accessed December 18, 2023. https://www.mlo-online.com/information-technology/data-management/article/53070653/overcoming-data-management-challenges-with-an-all-in-one-lims.
2. Moran RF, Lesica JP. An automated data management system for blood gas quality control. New Zealand J Med Lab Tech. 1983;37:28-55.
3. Moran RF. A Centralized Quality Control Data Processing and Management System for the Critical Care Laboratory. Market Issues and Operational Alternatives Leading to a Strategic Decision. Pacific Western University,1987.
4. Moran, RF. Computer Standards, Healthcare Management Briefs. 1990.
5. Vanderlinde RE, Schaffer R. Development of certified reference materials for the National Reference System for the Clinical Laboratory, Nat Comm Clin Lab Standards NRSCL3-A. Nat Comm Clin Lab Standards NRSCL3-A. Published online 1991.
6. Moran RF. Implications for the intensivist in relating pre-analytical treatment of patient blood samples to clinical reliability of electrolytes, PCO2 and total hemoglobin. West Pac Cong Crit Care Med. Published online 1993.
7. Howard, M and Moran, RF Implementing a complete laboratory information system (MediTech®, inc.) to ensure faster and more usable data in the right place and time. Unpublished, 1975.
8. Orazio D. Standardization of sodium and potassium ion-selective electrode systems to the flame photometric reference method - Approved Standard. Nat Comm Clin Lab Standards NCCLS: C29-A. Published online 1995.
9. Burnett R, Ehrmeyer SS, Moran RF. Blood gas and pH analysis and related measurements; Approved Guideline. Nat Comm Clin Lab Standards NCCLS C46-A. Published online 2001.
10. Moran RF. Point of Care vs. In: Central Lab Discrepancies: Getting the Message Across. JALM,1:05. ; 2017:595-597.
11. Podcast- Point of Care vs. In: Central Lab Discrepancies: Getting the Message Across. JALM. ; 2017.
12. Moran RF, Liesching TN. The ABC’s of ABG’s¿: A Cyclopedic Dictionary of the Testing Terms Used in Critical Care.; 2018.
13. Moran, RF, POC testing and reporting of sodium, and other small molecules need modified IFCC source/type designations to improve operational efficacy and for clinically accurate, unambiguous reporting. eJIFCC, December 2023.
About the Author

Robert F. Moran, PhD, FCCM, FIUPAC
is the Principal Scientist at mviSciences, a consulting and educational services organization and President of AccuTest™ Proficiency Testing Services. Dr. Moran served multiple terms on the NCCLS (Now CLSI) Board of Directors and was an active participant or chairholder in several of their blood gas and electrolyte standards-writing teams. Also active in clinical chemistry internationally, he is an appointed Fellow of the International Union of Pure and Applied Chemistry (FIUPAC). He is a retired professor of chemistry and physics from Wentworth Institute of Technology but remains active in consulting work and writing.