Connectivity and data management are vital for point-of-care testing
To take the test online go HERE. For more information, visit the Continuing Education tab.
LEARNING OBJECTIVES
Upon completion of this article, the reader will be able to:
1. Describe the role and responsibilities of a point-of-care-coordinator.
2. Discuss the uses and benefits of a data management system as it relates to operator, device, and result management.
3. List different types of reports a data management system can generate.
4. Discuss additional features and benefits of a data management system.
As the demand for point-of-care testing (POCT) continues to grow and the testing becomes more complex, it is more important than ever to be able to track and monitor all aspects of a POCT program and get all test results into the patient record in a timely manner. The pandemic made it clear that POCT is crucial and can take place just about anywhere — retail locations, campus parking lots, physician office labs, clinics, and mobile vans. Along with expanding locations, POCT has moved far beyond just glucose and urine dipsticks.1 We are now seeing devices that are usually considered ‘main lab’ moving out to these decentralized locations. No matter the location of the testing, the responsibility for the management of the testing, most often, still falls on laboratory personnel. Device connectivity and a robust data manager are imperative for a successful program by providing testing that is safe for patients and helping meet accreditation requirements.
Using connectivity to manage all device operators at the point-of-care
A point-of-care coordinator (POCC) (or technical consultant) has many responsibilities and one of the most important is operator management. As POCT expands, the coordinator has responsibility for educating, training, and assessing competency for anyone preforming testing outside of the main laboratory. This testing includes waived, non-waived, and in some case provider-performed microscopy (PPM) testing. Testing personnel can include certified nursing assistants, patient care technicians, nurses, respiratory therapists, perfusionists, and physicians.
A data manager is essential for the program to successfully track those who are qualified or certified to perform both waived and non-waived testing. The testing can be performed on a POCT device or be a manual, lateral flow-type test. Whether your lab is inspected by the College of American Pathologists (CAP), The Joint Commission (TJC), or Commission on Office Laboratory Accreditation (COLA), the medical director and the POCC are responsible for ensuring that testing personnel, often referred to as device operators, are trained and competent to perform testing.
A POCT data management system allows the POCC to enter all operators into a single electronic data base where they can give certifications for all testing done by each operator once initial training is complete. Qualifications such as diploma or license numbers can be documented within the system as having been verified.
Often, a data management system will allow the POCC to enable an automatic recertification for device operators based on very specific criteria that must be performed successfully for the operator to continue to perform testing. The criteria can include successful completion of quality control (QC), patient samples, and/or proficiency samples along with a quiz. Having a data management system also allows the POCC to document who directly observed operators’ performance of routine patient testing in order to meet regulatory requirements. The observation is then captured as part of the operator competency and can be used as criteria for automatic recertification.2
Using connectivity to manage all devices at the point-of-care
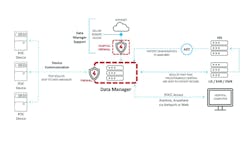
A POCT program can have many devices connected to the data manager (Figure 1). If the program had to connect the individual devices directly to a laboratory information system (LIS), the cost would be prohibitive. By connecting all POCT devices to a single data manager, only one interface is needed between the data manager and the LIS to get all the results to the patient record, unless there is an additional interface from the LIS to an electronic medical record (EMR). The data manager allows the POCC to view all devices and look for those that haven’t downloaded recently. In some cases, the POCC can sync a device with the click of the mouse. Depending on the device, the POCC can view error logs sent to the data manager and can also document device maintenance in the system. Being able to view the communication status and act upon the information ensures that patient results are being transmitted to the patient record in near real time.
Device communication – bidirectional and unidirectional
When devices are connected to the data manager in a bidirectional manner, results come from the device(s) into the system and the system can also send a list of valid, certified operators to the device. This provides true operator lock-out at the device, making it nearly impossible for an untrained, unapproved operator to perform testing.
For unidirectional devices and manual tests, only results are received by the data manager; operators aren’t ‘locked out’ from performing testing; however, the data manager can ‘flag’ results that are performed by an ‘uncertified operator,’ thus alerting the POCC of a potential issue. The POCC can still send those flagged results to the patient record if needed, but it gives the POCC the opportunity to be alerted and investigate the issue. In some cases, it may be that the operator was certified in the past, but the certification expired (certifications are usually for either six months or one year) and simply needs to perform his/her annual competency.
Using connectivity to manage all test results at the point of care
POCT programs range in size from a single institution with only glucose devices connected and 50 operators to a very large integrated delivery network with many institutions, device types, manual tests, and upwards of 5,000 to 10,000 operators. Along with operator management, having device connectivity and manual test entry through the data manager allows the POCC to manage all test results performed outside of the main lab. For large health networks, that translates to millions of POCT results per year.
The POCC is responsible for monitoring QC, patient, proficiency, and linearity results. For patient results, the POCC can enable flagging for critical results, results outside the analytical measurement range, and results outside the reference range. These results can still be sent to the patient record; however, the flagging or alerting allows the POCC to generate quality assurance reports, ensuring that testing personnel are following proper procedures for handling these types of results.
The POCT program can also use an admit/discharge/transfer (ADT) feed with the data manager. For some devices, the list of registered patients can be sent to the device. When an operator enters the patient ID at the device, the patient’s name and date of birth display on the device screen and must be confirmed prior to testing. When the ADT feed is used, this helps the POCC ensure that the correct patient ID is used at the device. If an unrecognized patient ID is entered, those results can also be flagged by the data manager and held for investigation by the POCC.3
Quality control results from all devices and internal and external controls for lateral flow tests must also be monitored and reviewed for compliance. For devices with quantitative results, the QC results download to the data management system, and the POCC can review individual results or view QC using Levey-Jennings (L-J) charts. L-J charts allow the reviewer to look for trends and shifts in QC and address issues accordingly (Figure 2). The L-J charts also show the statistical analysis for each device, including range, mean, standard deviation, and percent coefficient of variation (%CV). The reviewer can mark individual results and charts as reviewed and add additional review notes. The POCC can also handle any QC outliers and ensure QC passed prior to running patient tests.4
When asked for by an accreditation inspector or surveyor, the L-J charts are easily accessible to show proof of compliance. The POCC must also review qualitative QC to ensure the external controls are being performed successfully. For internal QC performed with the patient test, any failures can be flagged and those results held so that they do not go into the patient’s medical record. Failed QC results, whether quantitative or qualitative, can alert the POCC to possible issues with a device or the need for retraining if a specific operator continues to struggle with performing QC.
Some devices require linearity or calibration verification upon initial implementation, and most moderately complex devices require linearity every six months. By having POCT devices connected to a data manager, the operators can perform the linearity testing and those results download to the data manager for review by the POCC/TC. The linearity results are graphed, and statistical data is generated, including slope, Y-intercept, and percent correlation coefficient (Figure 3).
The POCC can review the linearity charts, verify reportable ranges and adjust a range if needed. In a large system with many connected devices requiring linearities, it’s crucial for the POCC to be able to have one place to review and store those records electronically, reducing printing costs and increasing efficiency. Again, verifying the performance of the device aids in ensuring accurate patient results.
A quality POCT program will also include the performance of proficiency testing (PT). Proficiency samples are run at the device and downloaded to the data manager. The results can be reviewed and stored electronically in the data management system. The POCC can mark the proficiency results as reviewed and indicate whether the testing was successful or unsuccessful once the assessment is received from the proficiency testing provider. The POCC uses the proficiency samples to monitor device performance and that the operator is handling samples correctly and performing testing correctly. A device may need to be taken out of service or an operator retrained in the event of failed proficiency. Reviewing and storing the proficiency results in a data management system eliminates printing and storage of paper documents and reduces the risk of lost or misplaced information.
Harnessing the management of manual or lateral flow-type test results, such as pregnancy, rapid strep, and fecal occult blood, has always been problematic. In the past, results were often manually written in the patient’s chart, with no documentation of passed internal QC, kit lot numbers, or who performed the test. The results were easily lost or overlooked when they were handwritten “somewhere” in the chart. A data management system that utilizes a manual test entry (MTE) feature allows the POCC to not only configure the result entry fields but also to track operators who are trained and certified to perform manual tests.
The certified operator enters the patient result, internal QC result, and if set up to do so, the kit lot numbers. Once entered, those results are evaluated by the system and if all information is entered correctly, the manual test result is sent to the patient electronic medical record (EMR). If the internal QC is invalid, the POCC can hold those results in the data manager so that the patient result is not sent to the EMR. The benefit of having all the documentation for the manual test and the result in the EMR far outweighs the minimal amount of training for the operator to use the MTE feature.
Using connectivity to manage test materials at the point of care
The POCC can enter all test materials, including strip/reagent/kit lot numbers, QC lots, linearity lots, and proficiency sample IDs in the data manager. The POCC can enter the test materials with manufacturer’s expiration date, along with acceptable ranges for QC and linearity. For devices that have bidirectional communication, the test materials are sent to the device, and the operators are not allowed to use materials that have not been entered in the system. The acceptable ranges are also sent to the device allowing the operator to be alerted by the device if QC or linearity results are out of range.
The data management system should also provide inventory tracking so that the POCC can plan ahead, making sure test materials are ordered in a timely manner. For unidirectional devices and manual tests, the system can be set up to flag results performed using new test materials that are not yet validated, and those results can be held or sent to the patient record. In this case, the POCC would be alerted and can investigate why the materials were used or can validate them and put them into use. Being able to track all lot numbers and inventory in the data management system eliminates the need for paper logs and ensures test materials are validated prior to use.
Reports manage key performance indicators
A POCT data management system will provide the ability for the POCC to generate various reports, which can be used as part of the laboratory’s key performance indicators. The POCC can also send reports to the various nursing unit managers or device operators as needed. Example reports may be ‘Critical values for a given time period’ or ‘Operator certifications expiring soon.’ The system should have a wide range of reporting capabilities that a POCC can use for quality assurance purposes and for communicating to testing personnel and unit managers. The reports can give insight into the overall health of the POCT program — what is going well and what areas may need improvement.
Additional features of a data management system at the point of care
Managing compliance and competency
A data management system may provide the POCC with the ability to create quizzes and assign them to the operators as part of their competency assessment. The successful completion of the quiz can be used as criteria for auto-recertification. The data manager may also be able to interface with an external learning management system (LMS) through a direct interface or through a file-based data import. Like the integrated quiz, the successful completion of the LMS course can be documented in the POCT data manager and used as criteria for recertification.5
Managing access to the data management system
Another feature of a data management system would be permission-based access to the system. A POCT program might not have 24-hour, 365-day coverage and the permission-based access allows the POCC to give access to the data management system to those who can assist with daily operations. For example, the program may have nurse educators who help train new personnel on the use of a glucose meter. The POCC can give system access to nurse educators to enter new operators and give glucose certifications in the system. The educators will have access to only the operator management function. Having additional staff resources access the system as needed for certain functions creates efficiency and uninterrupted service to the testing locations.
Using other programs to enhance the use of the data manager
Lightweight Directory Access Protocol (LDAP) or Active Directory is a great feature that can be used with the data management system. Manually entering new operators is very time consuming, especially with the current staffing issues found nationwide. This feature provides a means for the operators to be automatically added, edited, or removed from the data manager. The Active Directory that may already exist at an institution can be set to sync with the POCT data manager to add new operators to the system based on specific criteria. The sync can also remove operators who no longer work in the facility or network (such as travel or temporary staff) from the system, adding another layer of security to the program.
Conclusion
The pandemic has accelerated the use of POCT. Many clinicians who had not performed lab testing began performing COVID-19 and other types of tests. A data management system can ensure the newly implemented point-of-care tests continue to support the value-based healthcare provided.
As you can see, the POCT program can be quite complex and requires a multidisciplinary approach, including laboratory, nursing, respiratory therapy, and physicians. Having a POC data management system is vital for maintaining an inspection-ready program, reducing the workload on staff, increasing program efficiency, and improving patient safety.
References
- Point-of-care testing. Testing.com. Published January 27, 2021. Accessed June 21, 2022. https://www.testing.com/articles/point-of-care-testing/.
- Dyhdalo KS, Howanitz PJ, Wilkinson DS, Souers RJ, Jones BA. Documentation of quality control and operator training at point-of-care testing: a College of American Pathologists Q-Probes study of 106 institutions. Arch Pathol Lab Med. 2014;138(11):1444-1448. doi:10.5858/arpa.2013-0552-CP.
- Jones BA, Meier FA. Patient safety in point-of-care testing. Clin Lab Med. 2004;24(4):997-1022. doi:10.1016/j.cll.2004.06.001.
- Callender S. Point-of-care testing: ensuring patient safety through quality control. Biomed Sci. Accessed June 21, 2002. https://www.aims.org.au/documents/item/643.
- ISO 22870:2016. Point-of-care testing (POCT) — Requirements for quality and competence. 2016; 2.
Karen Carver, MT, Karen is a former Point-of-Care Coordinator and is now the ARDx Informatics Global Product Manager for RALS.
Zach Fortuna, MLS, CPP, Zach is a Marketing Specialist with ARDx Informatics. Prior to joining the RALS team, he was a Quality, Compliance, and Point-of-Care Coordinator at a large Academic Medical Center.
Carol Wise MT(ASCP), Carol has extensive experience in the laboratory from third shift generalist bench technologist to laboratory supervisor and much in between. She has covered the western United States training staff on laboratory devices including those for point-of-care for two different manufacturers.
Steven Valorz, is a Global Marketing Communications Manager with ARDx Informatics.
Understanding the difference between waived and non-waived testing:
Before the Clinical Laboratory Improvement Amendments of 1988 (CLIA), there was little to no testing being performed outside of the main clinical laboratory. Since CLIA, the number of CLIA-approved waived tests has risen dramatically, from 8 to over 1,400. People often use the terms “point-of-care testing” and “waived testing” synonymously. However, this is not an accurate statement. Point-of-care testing also includes non-waived testing, which is divided into two categories: moderate-complexity testing (including provider-performed microscopy) and high-complexity testing. It is important to understand the difference between waived and non-waived testing and how each applies to POCT. This will ensure that compliance with state and federal regulations and accreditation requirements are met.
CLIA waived testing: Tests with simple procedures that pose little chance of a negative outcome if the test is performed inaccurately/incorrectly.
Requirements:
- Must have a CLIA certificate of waiver.
- CLIA does not require policies for assessing personnel competency for waived testing.
- Even though CLIA has no specific requirements for personnel performing waived testing, there needs to be a way to ensure that patient test results are correct to assist in making an accurate patient diagnosis.
- Testing personnel must follow all manufacturers’ instructions.
- Usually requires staff to have minimal education, i.e., high school diploma
- If a laboratory is accredited by CAP or TJC, it may need to consult their standards.
- Some examples of waived tests:
- Bedside glucose
- Urinalysis
- Pregnancy
- Rapid strep
CLIA non-waived testing: Moderate complexity, high complexity, and provider-performed microscopy (PPM).
Requirements:
Personnel performing non-waived testing must have documented initial training by a technical consultant (TC) prior to patient testing. A TC must have a bachelor’s degree in a chemical, physical, biological, or clinical laboratory science or medical technology with at least two years’ experience in non-waived testing. The TC’s training and experience must include the discipline(s) in which the individual is providing consultation.
Evaluating and documenting competency of personnel responsible for testing is required at least semiannually during the first year the individual tests patient specimens (once at six months, once at one year). Thereafter, competency assessment must be performed at least annually through the following:
- Direct observations of routine patient test performance
- Monitoring the recording and reporting of test results
- Review of intermediate test results or worksheets, quality control records, proficiency testing results, and preventive maintenance records
- Direct observations of performance of instrument maintenance and function checks – quality control
- Assessment of test performance through testing previously analyzed specimens (proficiency samples)
- Assessment of problem-solving skills (quizzes)
Personnel performing non-waived testing are usually required to have higher education (beyond HS diploma) and in some cases licensure (Registered Nurse, Respiratory Therapist, Doctor of Osteopathy, or Medical Doctor).
Some examples of non-waived testing:
- Blood gas
- Activated clotting time (ACT)
- Fern test – PPM
About the Author

Steven Valorz
is a Global Marketing Communications Manager with ARDx Informatics.

Carol Wise MT(ASCP)
Carol has extensive experience in the laboratory from third shift generalist bench technologist to laboratory supervisor and much in between. She has covered the western United States training staff on laboratory devices including those for point-of-care for two different manufacturers.

Zach Fortuna, MLS, CPP
Zach is a Marketing Specialist with ARDx Informatics. Prior to joining the RALS team, he was a Quality, Compliance, and Point-of-Care Coordinator at a large Academic Medical Center

Karen Carver, MT
Karen is a former Point-of-Care Coordinator and is now the ARDx Informatics Global Product Manager for RALS.