The digital footprint in transfusion medicine and the potential for vein-to-vein management
The digital footprint from physician order entry to the Transfusion Service, through the various decision matrices within the blood supply chain, has not progressed as fast as those in other ancillary healthcare support areas (for example, pharmacy). This may change with the evolving impact of Integrated Delivery Networks (IDN), Clinical Integrated Networks (CIN), and Accountable Care Organizations (ACO) on modernizing efficient healthcare delivery by placing requirements on blood suppliers.
Numerous technological advances in immunohematology and blood banking have been made in the last 70 years, but the most significant change has been the acceptance of transfusion medicine (TM) as a medical discipline focusing on the patient to determine appropriate therapy. In the early years of this century, an economic downturn in the United States placed greater emphasis on patient blood management (PBM) and cost recovery, putting a damper on the nation’s blood system. During this time, TM refocused on the patient through therapeutic guidelines to ensure that every blood product transfusion was appropriate and, if possible, that alternate therapies were considered to reduce risk to the patient. As a result, blood usage, especially red cell transfusion, has decreased over the last 10 years. Reimbursement coverage, “never event,” and penalties for poor quality healthcare performers are shaping a drive for a better digital footprint, especially in transfusion medicine.
Going LEAN
As integrated health delivery evolves, efficient suppliers, including blood establishments, must become LEAN and practice healthcare Kaizen (otherwise known as, “continuous improvement”). LEAN has often been inappropriately branded by some as Less Employees Are Needed, but that is not the case as in most Kaizen approaches. Efficiency is the primary goal, but not necessarily at the expense of staff. A LEAN approach which embraces stewardship is key. That is, driving efficiency, safety, patient satisfaction and lower cost through a better blood safety digital footprint.
Errors in blood banks and blood centers are very rare, as most facilities overcompensate based on risk mitigation and build numerous checks in the system. The greatest risks are known to be “clerical,” and most happen post-blood bank. That is, most errors occur either by or within the healthcare facility with “wrong blood in tube” for specimen testing and wrong blood administered at the bedside.1 ABO blood group system incompatibility and errors resulting in transfusion adverse events are among the “never events” not covered by reimbursement, thus a financial liability of the hospital.2
With today’s emphasis on stewardship of the healthcare dollar and the constraints of reimbursement, the question of how to create healthcare efficiency in a managed-cost environment is crucial. The blood supply chain, including blood establishments and TM, is not solely a supplier of blood products as a commodity for the healthcare system but a value service providing the best product for the appropriate patient at the appropriate time, within a scheduled cost structure. The future of the blood supply chain to the healthcare system will be dependent on LEAN operations providing value through an integrated blood safety and availability digital network.
Transparency, V2V, RFID
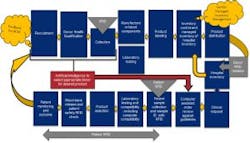
To achieve this, the blood bank digital footprint, either integrated or separate from the hospital information system (HIS), must become more transparent in data sharing, including supply chain management within the blood establishment. A shift to a transparent inventory has the potential to move the transfusion services blood supply within the IDN (integrated delivery network) to a vendor-managed inventory within the hospital. Transparency is critical to build the confidence of the IDN that its needs will be met for unexpected emergencies. A vein-to-vein (V2V) digital footprint with automation is needed to ensure both blood safety and availability (Figure 1). Acceptance of a blood safety and availability digital footprint is dependent on an “open” architecture based on common industry standards of data elements for all essential processes within the blood bank supply chain and to its final administration to the patient. Of course, cybersecurity will be of prime concern, and protection of patient/donor information will be paramount.
A V2V digital footprint within TM will require processes that may be facilitated by artificial intelligence (AI). This should include donor recruitment and selection as well as data obtained during the collection process. Checks and balances among expected needs as well as automation elements for greater efficiency are envisioned in component preparation, testing, labeling, and distribution. Management of the blood component, its labelling, and the specimens for testing, could be managed through radio frequency identification (RFID). Automating current manual processes with “minimal manual touch” is envisioned with RFID.
For example, imagine the digital footprint using automation and RFID to direct specimens for centrifugation or directly delivered to an instrument as whole blood. Imagine replacing multiple-person labeling practices compliant with Good Manufacturing Practices (GMP). The digital footprint could even streamline blood product ordering and establish logistics for delivery efficiency, ensuring the right blood at the right time to the correct location, all while reducing technical manpower and errors.
On the hospital side, the digital footprint could reduce errors when completing a physician order using AI and RFID for specimen identification and appropriate tracking. Reducing specimen labeling errors and errors of transfusion potential could create significant financial savings through an integrated V2V system. The future for TM within a changing healthcare system depends on defining a digital footprint involving both the supplier and the healthcare provider.
REFERENCES
- Bolton-Maggs P. SHOT conference report 2016: serious hazards. Transfusion Medicine. 2016;28:401-405.
- Castellucci M. New data from CMS’ hospital-acquired condition program have analysts questioning value. Modern Healthcare. 2017.
About the Author