Testing the performance of fully automated blood cell washers in pretransfusion compatibility testing
The transfusion of donor red blood cells (RBCs) that are incompatible with the recipient’s blood type can lead to immune-mediated hemolytic transfusion reaction, in which the recipient’s immune system produces antibodies that destroy the donor cells. This can lead to adverse symptoms including fever, dyspnea, hypotension, uncontrollable bleeding, and pain. In severe cases, acute renal failure may occur, which can be fatal.
To avoid the harmful consequences of these hemolytic transfusion reactions, and to ensure that donor RBCs survive in the recipient, blood banks and hospital laboratories routinely perform pretransfusion compatibility testing.1 However, prior to performing these tests, the donor RBCs must be thoroughly washed with physiologic saline to remove unwanted components, such as residual plasma and cellular debris, which could otherwise impair the reliability and reproducibility of the test results.
Given their importance in compatibility testing and patient safety, cell washing technologies have undergone considerable advancement in recent years, with traditional manual cell washing methods having been replaced by automated cell washing centrifuges. More recently, novel fully automated systems have been developed to further improve the quality and efficiency of cell washing. Here, we review the advantages that these advanced systems offer, focusing on a case study in which the performance of a new fully automated system was tested and compared with a more traditional system, to determine if use of newer systems can potentially enhance compatibility tests to better protect patients.
Pretransfusion compatibility testing
In blood banks and clinical laboratories, pretransfusion compatibility assessments typically involve two types of tests: the antibody screen test and the direct antiglobulin test (DAT). Each test has one or more purposes and a varying degree of importance in different situations.
The antibody screen test is generally performed on patients requiring blood transfusion or with suspected transfusion reactions, as well as on pregnant women and on blood/plasma donors. The test detects the presence and amounts of unexpected antibodies by observing the degree of agglutination, which is a process that occurs when an antigen is mixed with its corresponding antibody.
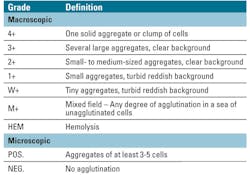
The antibody screen test involves incubating the patient’s plasma with commercially available RBCs, in the presence of low-ionic strength solutions (LISS) or polyethylene glycol (PEG), which are used to optimize agglutination reactions.2 Anti-human globulin (AHG) is then added to produce an antigen-antibody reaction. If no agglutination is observed (negative antibody screen), then it can be concluded that no significant antibodies are present. If any degree of agglutination is found, this indicates a positive antibody screen, which requires the identification of the antibodies. Following a positive antibody screen, the degree of agglutination is graded at both macroscopic and microscopic levels to indicate the amount of antigen/antibody present.
The other main type of compatibility test, the DAT, is typically used to diagnose causes of hemolysis, such as transfusion-related hemolysis, hemolytic disease of the fetus and newborn, autoimmune hemolytic anemia, and drug-induced immune hemolysis.3 The DAT detects whether the RBCs have surface-bound immunoglobulin G (IgG) antibodies and/or complement. The procedure involves incubating the patient’s RBCs with the AHG reagent, before centrifuging the RBC suspension to produce a pellet or dry cell button that is examined for agglutination. A positive DAT means that the patient’s RBCs are coated with antibodies, while a negative DAT indicates that there are no detectable antibodies attached to the patient’s RBCs.
Blood cell washing techniques
In both antibody screening tests and the DAT, the patient’s RBCs must first be washed in physiologic saline to remove unwanted components such as plasma, plasma protein, microaggregates, cytokines, and unwanted antibodies, as these can interfere with the test procedure and produce inaccurate results. Improper washing or under-washing of RBCs is a potential source of error in compatibility testing, having been identified as one of the most common causes of false negative or invalid test results.3
For example, human serum naturally contains IgG antibodies to any disease we have become immune to, or vaccinated against. As such, failure to sufficiently wash the RBCs before performing DAT may allow residual unbound IgG antibodies to remain in the tube, which then absorb and neutralize the AHG reagent. The cell washing process can also help to remove protein agglutination and some aged RBC segments to avoid test interference, as well as eliminate lactic acid that accumulates on the RBCs for better preservation.4
Traditional cell washing methods have used manual centrifugation. Typically, the clinician squeezes saline into the test tubes containing the sample, which are then placed into a serologic centrifuge. After centrifugation, the clinician decants the saline or removes it using a pipette or aspiration equipment, leaving the cell button intact for analysis. Not only is this a laborious and time-consuming process, but it has been suggested that manual washing poses a risk of contaminating the product due to human error, as well as the elution of low-affinity antibodies that interfere with the antigen-reagent/antibody reaction.5
Recently, more advanced, automated cell washing centrifuges are removing the need for these manual procedures. Automation has the advantage of improving the quality of cell washing by minimizing human errors and interference by unbound antibodies.5 Moreover, as automation has advanced in recent years, novel, fully automated cell washing systems have been developed to further improve cell washing quality, efficiency, and ease of operation.
Such systems can speed up the washing process to increase lab efficiencies, provide better saline decanting to minimize sample residue in the chamber, and optimize pelleting performance. In a recent study, the cell washing performance of a fully automated system was assessed to determine its suitability and potential impact on cell washing quality and reproducibility.
Case study: fully automated vs. manual
The study involved comparing the performance of a fully automated system to a more traditional model in pretransfusion compatibility tests, including an antibody screen test (using both PEG and LISS reagents) and a DAT. The pass criteria for both tests was based on a grading system (from 0 to 4+) of the degree of RBC agglutination (Table 1, Figure 1). An acceptable pass was when the reaction results were within one grade of the two cell washers, and an unacceptable pass was when the reaction results were greater than one reaction grade difference between the two cell washers.
Antibody screening tests
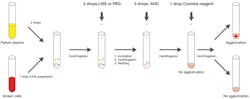
Using patient plasma samples, a total of 25 antibody screens using PEG and a total of 25 antibody screens using LISS were carried out following the same procedure (Figure 2). After preparation and centrifugation of the cell mixture (containing two drops of patient plasma plus one drop of screening cells), two drops of either LISS or PEG were added to the tubes, which were then incubated in a heat block at 37 degrees C for 15 to 30 minutes to allow the antibody to bind to any antigens on the reagent RBCs.
Following incubation, the cells were centrifuged, then resuspended by gentle agitation and examined for agglutination or hemolysis. The cells were carefully washed using either the fully automated or traditional system to remove unbound IgG, and to avoid their neutralizing the AHG reagent. Two drops of the AHG reagent were then added to the dry cell button in the tubes, before being centrifuged and resuspended by agitation and read macroscopically. If the test was negative, one drop of Coombs reagent was added to the cells, which were then centrifuged and analyzed for agglutination.
Direct antiglobulin test
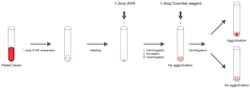
A total of 25 patient EDTA-preserved blood samples underwent a DAT. Preparation of the cells involved adding two drops of the cells to each tube, which were then washed using either the automated or traditional cell washing system to remove residual plasma and cellular debris. After washing, the cells were resuspended in 2 mL saline to obtain a three percent to five percent suspension, which was compared with a commercially prepared three percent to five percent RBC suspension.
After cell preparation, one drop of the suspension was added to each tube, which were subjected to the same procedure (Figure 3). The cells were washed with one of the two cell washers to remove any unbound IgG. After adding one drop of AHG to the dry cell button, the tubes were centrifuged, then resuspended by agitation and read for agglutination or hemolysis. The tubes were then incubated for five minutes, centrifuged again, and resuspended by gentle agitation and examined macroscopically. One drop of Coombs control cells was added to all negative tests, before being centrifuged and
examined for agglutination.
Results and conclusions
For all procedures, the cell button was consistent through testing and no hemolysis was observed. Of the 25 LISS antibody screening tests performed, 19 (76 percent) had the same reaction results for both the fully automated and traditional cell washers, and six samples had one reaction grade difference between the two cell washers. In addition, of the 25 PEG antibody screens, 22 (88 percent) had the same reaction, and three samples had one reaction grade difference between the two cell washers. Of the 25 DATs, 20 (80 percent) had the same reaction results for both cell washers, and five samples had one reaction difference between the two washers. As such, the test concluded that the automated system performs with reproducibility and in the acceptable criteria, without impacting quality.
Blood banks and clinical laboratories that perform pretransfusion compatibility testing must ensure that patient RBCs are thoroughly washed before being tested for their compatibility. This is to minimize the risk of contaminants interfering with the validity of the results, ensuring that blood transfusions can be performed safely.
Cell washing can, however, be a time-consuming task, and throughput and efficiency are key to any clinical lab. Recent technological advances have seen fully automated cell washers being introduced to streamline the washing process with faster cycle times. Data presented here support use of such systems, demonstrating that they provide results that are of the same quality as results provided by traditional systems. This ensures that pretransfusion compatibility tests are robust and reproducible, providing patients with the highest level of protection against harmful hemolytic transfusion reactions.
REFERENCES
- Shulman IA, Downes KA, Sazama K, Maffei LM. Pretransfusion compatibility testing for red blood cell administration. Curr Opin Hematol. 2001;8(6):397-404.
- Shirey RS, Boyd JS, Ness PM. Polyethylene glycol versus low-ionic-strength solution in pretransfusion testing: a blinded comparison study. Transfusion. 1994;34(5):368-370.
- Zantek ND, Koepsell SA, Tharp Jr. DR, Cohn CS. The direct antiglobulin test: a critical step in the evaluation of hemolysis. Am J Hematol. 2012;87(7):707-709.
- Yu Y, Ma C, Feng Q, et al. Establishment and performance assessment of preparation technology of internal quality control products for blood transfusion compatibility testing. Exp Ther Med. 2013;5(5):1466-1470.
- Gupte, SC, Automation in blood centre: Its impact on blood safety, Asian J Transfus Sci. 2015;9(Suppl 1):S6-S10.
Romana Hinz serves as Global Product Manager/Application Scientist for Centrifuges for Thermo Fisher Scientific.
Trace Bates serves as Inside Sales Representative for Thermo Fisher Scientific.