Benefits of an instrument-compatible capillary blood collection microtube
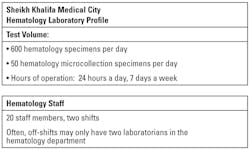
Sheikh Khalifa Medical City (SKMC) provides a network of comprehensive healthcare services for the Island of Abu Dhabi, United Arab Emirates, which include acute care facilities as well as tertiary surgical and medical services. The laboratory processes 600 hematology specimens per day with 50 pediatric specimens.
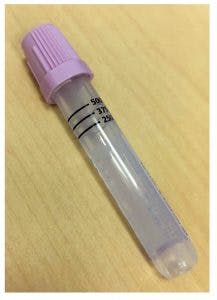
At SKMC, pediatric specimens are currently collected into a capillary blood collection microtube, obtained mainly from infants and newborns. In adults, specimens are collected in capillary tubes when venous collection is difficult. The accessioning, labeling, and testing of specimens collected in capillary tubes pose many challenges due to the small size of containers. The inability to properly affix a full-sized barcode label at the bedside can cause specimen labeling errors. The specimens might arrive in the hematology laboratory without a label or even mislabeled. Often, the barcode label is wrapped around the tube, which makes it difficult to read the patient’s information. Specimens with missing or illegible patient information are rejected and have to be re-collected, which contributes to additional work for healthcare personnel and subsequent delays in testing. Typically, there are restrictions on the amount of blood collected from these patients, and thus collection of a second specimen is problematic. In the context of these issues, hospitals and laboratory leadership try to find ways to improve service and workflow efficiency.
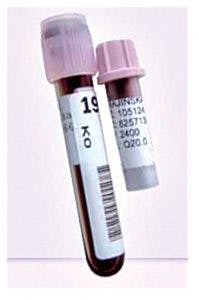
The capillary blood collection microtube with K2EDTA is used for the collection, transport, storage, and automated processing of capillary blood specimens for hematology testing. The instrument-compatible microtube has the same outer dimensions as a venous collection tube (13 x 75 mm) with a false bottom, which facilitates the collection of small volumes (maximum of 500 μL) of blood and the application of a full barcode specimen label. The tube has a pierceable cap, which allows it to be processed in the automated mode on hematology analyzers, thereby, minimizing laboratorians’ hands-on time in processing capillary specimens.
Conversion from the current equipment to the new microtube may enable the hematology lab to further improve efficiency and potentially reduce specimen labeling errors. Depending on lab specifics, conversion may result in significant time savings.
Facility analysis
Table 1 shows the SKMC hematology laboratory profile. This analysis was performed to evaluate the current microcollection process performed with the present equipment (Figure 1) and to determine if conversion to the new microtube (Figure 2) may aid in improving workflow. Methodically mapping the current procedure enabled identification of areas of waste that negatively impacted the process. Then, the processing was streamlined without compromising the essential collection steps or specimen quality.
Current workflow processes
Key findings: routing of specimens. The majority of specimens are received from the Pediatric Intensive Care Unit (PICU) between 3 am and 4 am, with additional specimens arriving throughout the day. During this period, these specimens are received in a batch of 10 to 15 tubes.
Current labeling issues. When specimens are received in reception, they are checked for labeling. If the tube is not labeled or is mislabeled, the specimen is rejected immediately and the nurse is then contacted to re-collect the specimen.
Presence of microclots. Tubes are inverted and checked for microclots manually, one by one. If a clot is present, a marker note is made on the label and the nurse is contacted to re-collect the specimen. The clot checking process includes the following steps:
- The cap is removed.
- Wooden sticks are used to check for clots.
- If clotted, the tube is recapped and is rejected, and the nurse is contacted to re-collect.
- If not clotted, the tube is recapped and is placed in the instrument running rack.
Specimen storage. After processing, the capillary tubes are stored in a box; the venous tubes are stored in tube racks. Retrieval of specimens from a box is more time-consuming than when the tubes are placed on a rack.
Specimen collection process flow. Hands-on (manual processing) capillary tubes (Table 2):
- Specimens are received.
- Tubes are uncapped.
- Tubes are checked for clots.
- Tubes are recapped.
- The instrument is set on manual mode.
- The specimen ID number on the barcode label on each tube is manually scanned using the instrument barcode wand or is manually entered into the instrument.
- Blood specimens in each capillary tube are manually aspirated.
- The tube is then placed on a rack for result verification.
Hands-on (automated processing) instrument-compatible microtube:
- Specimens are received.
- Tubes are uncapped.
- Tubes are checked for clots.
- Tubes are recapped.
- Tubes are placed on rack.Labels are aligned/specimens are processed in the automated mode on the analyzer.
The hands-on time required for laboratorians to process the two tubes was evaluated on three separate days (Table 2).
On Day 1, a batch of nine capillary tubes was processed (two specimens were rejected due to clotting, seven specimens were analyzed); hands-on time required was recorded. The hands-on time included the time required to check all nine tubes for clots and for the manual processing of seven tubes on the hematology analyzer.
On Day 2, a batch of 15 capillary tubes and 15 instrument-compatible microtubes was processed, and on Day 3, a batch of 20 capillary tubes and 20 instrument-compatible tubes was processed. Hands-on time for processing each batch was recorded for each day.
Results
The hands-on time required to process each batch of tubes is presented in Table 2. When processing a batch of capillary tubes, the laboratorian first checked each tube in the batch for microclots. If clots were observed, the tube was discarded and not processed further. If no clots were observed, each tube was sequentially manually processed. The hands-on time was calculated for each batch of tubes using a stopwatch. The stopwatch was started from the time the staff member checked the first tube for clots until all tubes were manually processed on the hematology analyzer.
When processing a batch of the instrument-compatible microtubes, the laboratorian first checked each tube for microclots and placed the tubes in an instrument rack. The rack was then placed on the instrument for automated processing. The hands-on time was calculated for each batch of tubes using a stopwatch. The stopwatch was started from the time the laboratorian checked the first tube for clots until all tubes were checked for clots, placed the tubes on the instrument tube rack, and then placed the rack on the analyzer for automated processing.
This analysis showed that a significant amount of laboratorians’ hands-on time is saved when a batch of the instrument-compatible microtube is processed compared with a same sized batch of capillary tubes—an average savings of 49 seconds per tube or an average savings of eight minutes per batch of 10 tubes.
When processing current microtube specimens in a batch, a considerable amount of laboratorians’ time is spent waiting for the instrument to complete specimen analysis on a tube and to reset, before the second tube can be manually aspirated. This time savings is especially impactful during peak hours when large numbers of specimens are received in the laboratory and there is only one laboratorian in the laboratory. Less hands-on time spent by the laboratorian processing a microtube specimen in the instrument-compatible microtube allows him or her to focus on other technical aspects, such as performing manual differential counts or other value-added work.
Conclusion
Converting to the instrument-compatible microtube can potentially offer the following benefits for the laboratory:
- An 80 percent reduction in the laboratorian’s hands-on time, enabling the technical staff to focus on other value-added tasks.
- Reduced specimen identification errors, since a full-sized label can be placed on the tube.
- Establishment of a simplified and uniform process for all received specimens without workflow interruptions for manual processing.
- Improved laboratory efficiency and turnaround time.
- Better storage (tube rack versus box) and retrieval of processed specimens, facilitated by the 13 x 75 mm tube configuration.
- Smoother transition between shifts, since specimen processing can be completed more rapidly and less work may remain for the next shift personnel.
Disclosure: The authors declare no conflict of interest. This study was supported by Becton Dickinson company in the United Arab Emirates.
Dima Fouad Yassin, MT (Bsc), CPHQ, Senior Supervisor, serves in the Department of Pathology and Laboratory Medicine, Sheikh Khalifa Medical City, Abu Dhabi, United Arab Emirates.
Mousa A. Al-Abbadi, MD, FCAP, CPE, CPHQ, FIAC, serves in the Department of Pathology and Laboratory Medicine, Sheikh Khalifa Medical City, Abu Dhabi, United Arab Emirates.