Decades ago, urinalysis was an important tool in the clinical diagnostic toolkit, and it was widely understood to provide the clinician a wealth of information on a patient’s condition.1 It is no secret, however, that urinalysis has been a bit behind in terms of technological advancements, remaining largely unchanged since its early days. With the advent of newer, more descriptive, and more specific serum tests and methodologies, the focus on urinalysis has slipped away, along with precious capital budget funds and educational focus. But the underappreciated discipline just might be primed for a resurgence of sorts, and, as more attention is paid to the quality of test results obtained and the resulting impact on patient care, the oldest form of clinical testing could still offer advances to improve patient care.2
The last major change to the urinalysis testing model was the automation of urine sediment analysis and the associated reduction in the need to perform the tedious, messy, and time-consuming manual microscopic review. This shift was mandated in part by the first wave of experienced medical technologists hitting retirement age, leaving the lab, and taking years of clinical microscopy experience with them. New medical technology graduates, raised on automation and computers and with comparatively little exposure to traditional manual methods, welcomed the new and easier way of performing urine sediment testing that virtually eliminated the need for the old-school manual slide review. For nephrologists, urologists, and other clinicians, CLIA regulations restricted the ability to perform microscopic urine sediment analysis outside of clinical laboratories, so their ability to accurately read and interpret urine sediment findings waned as well.
The search for biomarkers
To compensate for this decline in skillsets, the nephrology community began researching a “renal troponin,” that is, the biomarker(s) that would help clinicians quickly and more accurately diagnose kidney disease. While the research has uncovered some unique biomarkers with great potential—KIM-1 (kidney injury molecule 1), Cystatin-C, NGAL (neutrophil gelatinase associated lipocalin) and others—the markers do not always provide a consistent indication of the origin of the injury, so abnormal results can point to a range of conditions.3 Because of this, the medical community has yet to adopt any one marker as the acute kidney injury (AKI) marker of choice. But the potential for routine ordering patterns incorporating urinary biomarkers conjures images of “wet” urine chemistry testing platforms, using ELISA methodology to quantitate urinary biomarkers in conjunction with standard urine sediment testing, to offer true one-stop AKI diagnostic shopping.3
Any urinary biomarker assay developed for the market should be easy and inexpensive to perform, and not subject to interference from other urinary chemistries. The best biomarkers—think of troponin, TSH or HbA1c—are also stable, specific, and reproducible, providing a consistent clinical message regarding the patient’s condition.
When viewed this way, it is not a stretch to say that an effective urinary biomarker is already available to clinicians in the form of urine sediment analysis. Like any other organ-specific marker, urine sediment analysis is a real-time biological indicator of processes within the kidney, and its greatest strength lies in its ability to point to the anatomic origins of AKI. When combined with urine test strip chemistry results, it can provide information on the kidney structures (glomerulus or tubules) directly affected by disease.3
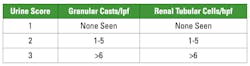
To date, with automated methods, elements in the urine sediment have been reported only as flags, with operator follow-up required before the final result is released. Looking at urine sediment analysis as a disease biomarker, there may be evidence to support quantitation of some of those flagged parameters. Nephrology literature suggests that a urine sediment “score” (Figure 1) consisting of the number of renal tubular epithelial cells per high power field and granular casts per low power field can be used to differentiate hospital-acquired AKI from acute tubular necrosis (ATN) and acute renal failure caused by a sudden reduction in blood flow to the kidney (pre-renal AKI). A urine sediment score of ≥2 has a positive predictive value of 100 percent for ATN. A score of ≥3 can be an indication of worsening AKI and probable patient mortality.3
Further drivers of urinalysis’ resurgence
The evaluation of urinary red cell morphology may also help drive the resurgence of urinalysis. Studies of patients presenting with persistent microscopic hematuria of unknown origin (PMHUO) indicate that those patients with glomerular disease exhibit deformed (dysmorphic) red cells, whereas patients with disease of the lower urinary tract express red cells that are normal and homogenous in shape and size.4 In a separate study, 14 of 16 (87.5 percent) study subjects with PMHUO who underwent kidney biopsy were found to have glomerular disease.3 Based on these findings, the Milan Clinical and Research Laboratory on Urinary Sediment performs an evaluation of urinary RBC morphology on all patients presenting with persistent microscopic hematuria of unknown origin before proceeding with more invasive testing. The hematuria is considered glomerular in origin when ≥40 percent of at least 100 urinary RBC are dysmorphic, or ≥5 percent are proven to be acanthocytes via phase contrast microscopy.4
And let’s not forget the significance of urinary bacteria, and the growing body of evidence that effective screening of routine urine samples for urinary tract infection can have a positive financial impact on hospitals while improving patient care by reducing unnecessary antibiotic use. Hospital-acquired urinary tract infections, especially those in catheterized patients, are considered sentinel events, and add an average of $1,000 to the care of each patient, for a total financial impact to hospitals of $450 million per year.5 The Centers for Medicare and Medicaid Services (CMS) will no longer reimburse any of the costs associated with the care of a patient who develops a urinary tract infection while hospitalized, so the potential impact to a hospital’s bottom line is even greater. Accurate bacteria and WBC counts from an automated urine sediment analyzer could lead to time and money savings for hospitals by demonstrating that the UTI was present at the time of admission. In the outpatient setting, an effective screen can reduce prescriptions for antibiotics for those patients without an active infection.
The view from here
In a few years—or perhaps more than a few, but in the foreseeable future—urine may again become the specimen of choice for detecting cancer and other diseases with a genetic component,2 with the next generation of urinalysis analyzers looking more like their immunoassay or chemistry cousins than the traditional urinalysis platform. New parameters and new clinical applications could be introduced to the market as study evidence builds to support the routine clinical use of urinary biomarkers. In the meantime, advances in automated urine sediment analysis could provide benefits to clinicians that were previously only seen with traditional manual microscopy, bridging the gap between the old and the new, and bringing urinalysis back into the spotlight.
REFERENCES
- Griswold A. In urinalysis, automated microscopy making the difference. CAP Today. March 2014.
- Halasey S. Urinalysis gets a new look. CAP Today. May 2016.
- Perazella M. The urine sediment as a biomarker of kidney disease. Am J Kidney Dis. 2015;66(5):748-755.
- Becker G, Garigali G, Fogazzi B. Advances in urine microscopy. Am J Kidney Dis. 2016; 67(6):954-964.
- Fuchs MA, Sexton DJ, Thornlow DK, Champagne MT. Evaluation of an evidence-based, nurse-driven checklist to prevent hospital-acquired catheter-associated urinary tract infections in intensive care units. Journal of Nursing Care Quality. 2011;26(2):101-109.
Leslie Williams, MT(ASCP), serves as Product Manager, Urinalysis, for Sysmex America, Inc.