Neurodegeneration is the underlying mechanism behind many diseases such as Alzheimer’s disease (AD), Parkinson’s disease (PD) and motor neuron diseases (MND), as well as cases of traumatic brain injury (TBI) and stroke. It is often difficult to identify neurodegeneration. Reliable biomarkers are critically needed to diagnose specific neurodegenerative diseases at an early stage, and help differentiate patients with overlapping clinical conditions. The classic biomarkers for AD are beta-amyloid (Ab) and tau, and their analyses in cerebrospinal fluid (CSF) are part of the 2011 National Institute on Aging and Alzheimer’s Association (NIA-AA) diagnostic guideline.1,2
With scientific advances in the field, the NIA-AA updated the 2011 guidelines and published a research framework in 2018. This framework lays emphasis on the diagnosis of AD using biomarkers, shifting disease definition from a syndromal to a biological construct in the living.3 Biomarkers in AD are grouped according to the amyloid, tau and neurodegeneration (ATN) classification system, which categorizes different biomarkers according to the underlying neuropathological change they measure, such as Ab deposition, tau aggregation and neurodegeneration, respectively.3,4 The research framework is currently intended for observational and interventional research, but not for routine diagnostics since the research framework is not a diagnostic criterion or guideline.3
Neurodegenerative process
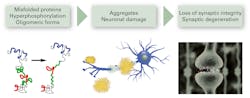
Neurodegeneration refers to any pathological condition that results in the progressive loss of structure or function of neurons. This process is regarded as a continuum that starts with misfolding of proteins due to various causes such as genetic mutations, RNA translational errors and abnormal post-translational protein modifications,5 that ultimately results in formation of neuro-toxic aggregates such as plaques and tangles (Figure 1). In AD, plaques consist of aggregates of the pathogenic protein Ab 1-42 (Ab42), which deposit extracellularly next to the nerve cell ends. In contrast, neurofibrillary tangles are made of tau proteins and are located inside nerve cells. All of these protein aggregates appear to acquire toxic properties damaging to neurons. As a consequence, there is a loss of synaptic integrity or degeneration of the synapses, leading to cognitive decline and other neurological symptoms.6
Alzheimer’s disease
Alzheimer’s disease is the most common cause of dementia in the elderly, and is observed in almost 70 percent of all dementia cases. AD is considered the sixth leading cause of death in the United States. Approximately 5.8 million Americans suffer from AD.7 People with AD experience various symptoms that change over time, depending on the extent of damage to neurons in different parts of the brain. Therefore, the disease is divided into three consecutive phases: the preclinical stage, the mild cognitive impairment (MCI) stage and the dementia stage.2 As the disease progresses, there is significant damage to the neurons causing cognitive decline and negatively impairing the individual’s memory, thinking and behavior. On average, the life expectancy after onset of symptoms is seven to 10 years in patients aged 60-70 years.8 Definitive diagnosis of AD is challenging and requires evidence of the neuropathological alterations in the brain. A diagnosis of probable AD is based on the clinical signs of memory loss and behavioral changes and the exclusion of possible reversible causes. Imaging techniques such as magnetic resonance imaging (MRI) or positron emission tomography (PET) for detection of specific protein aggregates are used to support differential diagnostics.9,10 Clinical studies and recent biomarker data indicate that the pathology of AD begins to accumulate at least 10-20 years before the appearance of cognitive symptoms. Currently, the “gold standard” for the definitive detection of amyloid-pathology in a patient’s brain is amyloid staining in postmortem autopsy brain tissue.11 Hence, there is an urgent need for pre-mortem biomarkers that demonstrate strong indications of amyloid pathology. Analysis of biomarkers in CSF aids diagnosis, especially in the early stages, and helps to discriminate AD from non-AD dementia patients.11 CSF biomarkers that support the diagnosis of AD include Ab42, Ab40, total tau (Ttau) and phosphorylated tau (P-tau).12
Beta amyloid
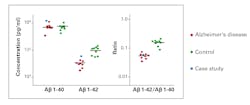
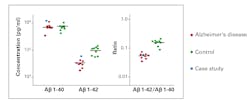
One of the core neuropathological characteristics of AD is the accumulation of Ab containing neuritic plaques in the brain parenchyma. Postmortem studies have indicated an inverse association between CSF Ab and amyloid plaque burden, caused by increased deposition of Ab42 in the brain.13 Therefore, individuals with AD show significantly decreased levels of Ab42, which is detectable at least five to 10 years before the onset of cognitive decline. Unlike Ab42, the values for Ab40 in CSF remain stable in persons with amyloid-pathology, reflecting its utility as a marker of the individual’s amyloid level. Many studies have reported better clinical performance with CSF Ab42/Ab40 ratio compared with CSF Ab42 alone for detecting Ab cortical deposition burden in prodromal AD.14-16 CSF Ab42/Ab40 ratio can differentiate AD from other dementia-related disorders (Figure 2), and can improve progression prediction in subjects with mild cognitive impairment (MCI). Determination of ratio increases the efficiency of early diagnosis compared to analysis of the individual biomarkers alone, and can contribute to delimitation from other dementia syndromes (e.g. vascular dementia, frontotemporal dementia). Furthermore, studies have reported high concordance between Ab42/Ab40 ratio and PET measurements in determining abnormal cortical Ab deposition.17 The overall prediction success rate increases from 83 percent to 93 percent when using the ratio compared with Ab42 alone. Therefore, CSF Ab42/Ab40 ratio is superior to Ab42 alone and can be used as an alternative to amyloid PET (imaging with an amyloid-specific contrast dye) to estimate Ab neural plaque density in adults with cognitive impairment who are being evaluated for AD.18 The major histopathological features of AD include not only deposition of amyloid plaques, but also accumulation of neurofibrillary tangles. In AD, tau becomes hyper- phosphorylated causing neuronal dysfunction. Consequently, a combination of release from neuronal injury and active secretion of tau proteins results in increased CSF levels (2-3 fold) of tau in mild-moderate AD patients compared to agematched controls. T-tau is a general marker of unspecific neuronal damage which occurs in AD, but also in other conditions such as stroke, traumatic brain injury, etc. In contrast, hyperphosphorylated tau such as P-tau(181), is an AD-specific marker indicating tauopathy.19 At EUROIMMUN, measurement of P-tau demonstrated a positive predictive value for AD at 88 percent and a high negative predictive value at 91 percent (Figure 3). The NIA-AA diagnostic recommendation for AD published in 2011 divided AD into three clinical entities: (1) preclinical, (2) MCI and (3) dementia stages.2 The 2018 updated research framework is established based on the definition of AD as a biological construct. The ATN classification system for biomarkers was introduced in 2016, and was designed using both CSF and imaging biomarkers in each group. It is possible to characterize the research participants using either biomarker alone or biomarker with imaging when available. “A” refers to aggregated Ab or associated pathologic state, which is measured by amyloid PET or CSF Ab42or Ab42/40 ratio, respectively. “T” refers to aggregated pathologic tau or associated pathologic state, which is measured by tau PET or CSF P-tau, respectively or tau PET (imaging with a tau-specific contrast dye). “N” refers to general biomarkers of neurodegeneration or neuronal injury including CSF T-tau, MRI and fluorodeoxyglucose PET.
Classic AD assays measure aggregates in CSF, which reflect the neuropathological changes in the brain. Synaptic protein biomarkers (see below), in contrast, provide a measurement of synaptic integrity and are assumed to be a more direct predictor of cognitive impairment. The ATN classification system is flexible since it is possible to add additional biomarkers that focus on neurodegeneration or synaptic integrity to the research framework as they become available.3,4,20 There is a direct correlation between synaptic dysfunction and memory disturbances including cognitive symptoms even at very early stages of AD. Synaptic proteins reflecting this pathophysiological process in the CSF could be useful biomarkers to monitor synaptic degeneration and, in turn, to monitor cognitive decline in AD. In the ATN classification system, all biomarkers that indicate neurodegeneration or neuronal injury in general are grouped into the “N” category. Neurogranin, Beta-secretase 1 (BACE1) neurofilaments, alpha-synuclein and other synaptic proteins are some of the promising biomarkers that belong to this category.3 Recent evidence shows that patients with MCI, as well as AD-related dementia, have significantly increased neurogranin levels compared with healthy controls. Additionally, CSF neurogranin levels are elevated in the prodromal stages of AD depicting synaptic injury as a measure of cognitive decline in AD.21 BACE1 is a pre-synaptic protein, whose levels are increased in AD patient brains.
When compared to the individual analytes, the ratio of neurogranin/BACE1 portrays significant correlation to cognitive loss with rapid decline in mini mental status examination scores (MMSE). A high ratio predicted a more rapid decline in MMSE scores. Therefore, the CSF neurogranin/BACE1 ratio could be potential progression markers of cognitive decline in AD.22 Assays for these biomarkers are currently used only in a research capacity. Studies are underway to determine the diagnostic and prognostic value of these parameters in a clinical setting.
The global burden of neurodegenerative diseases is increasing progressively, particularly in countries with aging populations. As of 2019, approximately 5.8 million Americans of all ages are living with Alzheimer’s dementia. By 2050, the number of people aged 65 and older with Alzheimer’s dementia in the U.S. is projected to increase to 13.8 million, straining health care systems.7 Due to the devastating nature of these diseases, early diagnosis is critical to enable therapeutic intervention and organization of adequate care. Therefore, there is an urgent need for reliable and robust biomarkers that support early diagnosis, differential diagnosis and prognosis in AD. The NIA-AA research framework stresses the diagnosis of A D using biomarkers, but is not based on the underlying clinical symptoms and signs. Currently, the biomarkers implemented in AD are invasive, as well as expensive. Therefore, the Alzheimer’s biomarker consortium groups are keen on developing less-invasive and less-expensive blood-based biomarkers that, along with genetic and clinical information, would support screening target populations at an early stage.
REFERENCES
- Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia. 2011;7(3):270-279.
- Jack CR, Albert MS, Knopman DS, et al. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia. 2011;7(3):257-262.
- Jack CR, Jr., Bennett DA, Blennow K, et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14(4):535-562.
- Jack CR, Jr., Bennett DA, Blennow K, et al. A/T/N: An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology. 2016;87(5):539-547.
- Fox LM, Yamamoto A. Chapter 7. Macroautophagy of Aggregation-Prone Proteins in Neurodegenerative Disease. Hayat MA, ed. Autophagy: Cancer, Other Pathologies, Inflammation, Immunity, Infection, and Aging. Amsterdam: Academic Press; 2015:117-137.
- Crews L, Masliah E. Molecular mechanisms of neurodegeneration in Alzheimer’s disease. Hum Mol Genet. 2010;19(R1):R12-R20.
- Association As. Alzheimer’s Disease Facts and Figures. 2019.
- Zanetti O, Solerte SB, Cantoni F. Life expectancy in Alzheimer’s disease (AD). Archives of Gerontology and Geriatrics. 2009;49:237-243.
- Aging NIA. How Is Alzheimer’s Disease Diagnosed? https://www.nia.nih.gov/health/how-alzheimers-disease-diagnosed. Published 2017. Accessed September 20, 2019.
- Small GW. Early diagnosis of Alzheimer’s disease: update on combining genetic and brain-imaging measures. Dialogues Clin Neurosci. 2000;2(3):241-246.
- Sutphen CL, Fagan AM, Holtzman DM. Progress update: fluid and imaging biomarkers in Alzheimer’s disease. Biological psychiatry. 2014;75(7):520-526.
- Blennow K, Hampel H, Weiner M, Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nature Reviews Neurology. 2010;6(3):131-144.
- Motter R, Vigo-Pelfrey C, Kholodenko D, et al. Reduction of b-amyloid peptide42 in the cerebrospinal fluid of patients with AD. Annals of Neurology. 1995;38(4):643-648.
- Pannee J, Portelius E, Minthon L, et al. Reference measurement procedure for CSF amyloid beta (Ab)1–42 and the CSF Ab1–42/Ab1–40 ratio – a cross-validation study against amyloid PET. Journal of Neurochemistry. 2016;139(4):651-658.
- Lewczuk P, N L, P S, JM M, J K. Amyloid-b 42/40 cerebrospinal fluid concentration ratio in the diagnostics of Alzheimer’s disease: validation of two novel assays. Journal of Alzheimer’s Disease. 2015;43(1):183-191.
- Janelidze S, Zetterberg H, Mattsson N, et al. CSF Ab42/Ab40 and Ab42/Ab38 ratios: better diagnostic markers of AD. Annals of clinical and translational neurology. 2016;3(3):154-165.
- Blennow K, Mattsson N, Scholl M, et al. Amyloid biomarkers in Alzheimer’s disease. Trends Pharmacol Sci. 2015;36(5):297-309.
- Janelidze S, Zetterberg H, Mattsson N, et al. CSF Abeta42/Abeta40 and Abeta42/Abeta38 ratios: better diagnostic markers of Alzheimer disease. Ann Clin Transl Neurol. 2016;3(3):154-165.
- Meredith Jr JE, Sankaranarayanan S, Guss V, et al. Characterization of Novel CSF Tau and ptau Biomarkers for Alzheimer’s Disease. PLOS ONE. 2013;8(10):e76523.
- Jack CR, Wiste HJ, Weigand SD, et al. Age-specific and sex-specific prevalence of cerebral b-amyloidosis, tauopathy, and neurodegeneration in cognitively unimpaired individuals aged 50–95 years: a cross-sectional study. The Lancet Neurology. 2017;16(6):435-444.
- Wang L, for the Alzheimer’s Disease Neuroimaging I. Association of cerebrospinal fluid Neurogranin with Alzheimer’s disease. Aging Clinical and Experimental Research. 2019;31(2):185-191.
- De Vos A, Struyfs H, Jacobs D, et al. The Cerebrospinal Fluid Neurogranin/BACE1 Ratio is a Potential Correlate of Cognitive Decline in Alzheimer’s Disease. J Alzheimers Dis. 2016;53(4):1523-1538.
About the Author

Iswariya Venkataraman, PhD
serves as Scientific Affairs Lead, EUROIMMUN U.S. She’s currently a member of the Alzheimer’s Disease Neuroimaging Initiative (ADNI) Private Partner Scientific Board (PPSB), an independent, pre-competitive forum for study-related scientific exchange in the field of neurodegeneration. During her doctoral studies in neuroscience, she identified novel auto-antigens in autoimmune hyper-excitability disorders.