Leukocyte alkaline phosphatase (LAP) by flow cytometry
Introduction
A Leukocyte Alkaline Phosphatase (LAP) score is still requested from the hematology laboratory as an aid in differential diagnosis in the peripheral hospital setting where molecular biology techniques are not readily available. The LAP score is usually decreased in chronic myelogenous leukemia and paroxysmal nocturnal hemoglobinuria, while it is increased in leukemoid reaction and polycythemia vera. Numerous other conditions may also increase or decrease the LAP score.1
Although the test is not specific for a particular disease, it is sometimes useful in confirming a suspected diagnosis or distinguishing between two conditions (e.g., CML and leukemoid reaction).
The test has classically been performed by a histochemical reaction2,3 on a fresh blood smear. The histochemical method is subjective, time consuming, and exposes laboratory workers to various chemical hazards.2,4 The use of flow cytometry to assess LAP was reported for research uses5,6 with non-commercial antibodies. A recent report of using a commercially available antibody discussed establishing normal LAP levels for individual hematology laboratories.7 The current study expanded on the use of a commercial primary antibody for clinical use by exploring different gating strategies, verifying assay conditions, and testing on new cases of CML or leukemoid reaction. A “LAP Index” of % positive x Median Fluorescent Intensity (MFI) was used after statistical analysis showed significant differences in this index between expected normal, high, and low LAP scores.
Materials and methods
The project was approved by the local ethics committee, and all participants signed informed consent forms. Fifteen healthy volunteers, two new CML patients, two leukocytosis/leukemoid reaction patients, and one treated CML patient were included.
Whole blood was rinsed three times in PBS + 0.5% BSA and incubated with a cocktail of CD16-FITC/ LAP-PE/CD14-ECD/ CD45-PC5 (LAP-PE from R & D Systems, all others Beckman Coulter) for 30 minutes at room temperature in the dark. Samples were hemolyzed and rinsed twice with PBS + 0.5% BSA and resuspended in 0.5 mL PBS. A Coulter EPICS XL-MCL was used for sample data acquisition. Kaluza software was used for data analysis.
Histochemical analysis of LAP activity was performed using the Sigma-Aldrich kit.8 Scoring was performed in duplicate by two experienced laboratory technicians.
The Mann-Whitney non-parametric test was used to compare normal, CML, and leukemoid results. The Kruskal-Wallis non-parametric test was used to compare the histochemical results with the flow cytometry results.
Results
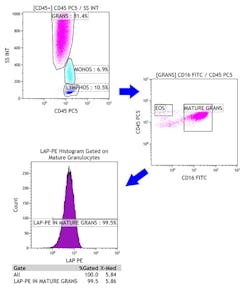
A gating strategy was established to measure LAP in mature granulocytes. Briefly, after gating on singlet events and gating out debris on FS/SSC (data not shown), CD45 vs. SSC groupings were established for granulocytes, monocytes, and lymphocytes. The granulocyte gate was then gated on CD16 positive events in a CD45 vs. CD16 dot plot for mature granulocytes. A histogram of LAP-PE on the mature granulocytes was then gated. Percent positive LAP-PE mature granulocytes and MFI were calculated from the histogram data (Figure 1).
Figure 1. Gating strategy.
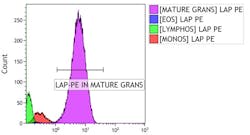
Non-neutrophils compared with FMO controls (data not shown) as an internal negative control. By using CD16 and CD14, mature neutrophils can be gated more clearly from monocytes, as well as cells more likely to be eosinophils (CD16-, higher CD45 in the granulocyte gate). In cases of PNH, CD15 can alternatively be used for gating on mature granulocytes (Figure 2).
Figure 2. Internal negative controls. By gating on CD45/SSC cell groupings, internal negative controls for LAP can be established. FMO controls showed similar results.
Time of incubation was tested and a decision made to keep the 30-minute incubation as in previous reports.7 Two rinses before staining were found to be sufficient for good peak resolution, and rinsing after staining and hemolysis was preferred. A comparison of EDTA vs. heparin anticoagulant showed that EDTA LAP Index numbers were consistently lower than the heparin preserved samples. Two different lysis methods showed similar results. A commercially available lysis method yielded stable results for 96 hours when preserved in 1% paraformaldehyde, where a laboratory specific method was less stable over the same period.
Discussion
In facilities with molecular biology diagnostics readily available, current diagnostic procedures can rely on the consistent association with the BCR-ABL1 fusion gene on the Philadelphia chromosome for differential diagnosis of chronic myelogenous leukemia (CML).9 In a more peripheral setting, this entails acquiring permissions for outsourcing the genetic testing, transport of the patient sample to the outsource facility, and an extended wait for results. The World Health Organization 2008 descriptions of CML, BCR-ABL1 positive, and chronic neutrophilic leukemia mention the markedly decreased neutrophil alkaline phosphatase of the chronic phase (CP) neutrophils.10 Hematologists and internal medicine specialists in the peripheral hospital setting still find the LAP test extremely useful in the diagnostic pathway to CML and other hematologic diseases; although not specific enough, it is sometimes useful in confirming a suspected diagnosis. A low LAP score with neutrophilia of unknown origin would justify the extra expense and trouble of outsourced molecular genetic testing. A high LAP score with no blast population present could direct the physicians to the differential diagnosis for leukemoid reactions.
The histochemical method of LAP scoring includes laboratory personnel exposure to formalin, a suspected carcinogen,4 methanol, and N,N-dimethylformamide, both irritants. The quality of the final stained smear depends on the amount of time between sampling and fixation and proper storage of the fixated sample before staining. The LAP score by light microscopy is considered a very subjective score.11 With only 100 mature neutrophils counted, the statistical significance of the LAP score is low. These safety and technical considerations are largely ameliorated by using flow cytometry for LAP index.
The flow cytometry assay counts tens of thousands of cells in a matter of minutes, includes negative internal controls, and results can be interpreted easily with preset gating strategies.7 Positive controls can be added by selecting samples expected to be high, such as those from maternity ward admission.12 Local normal ranges can be established by the laboratory. For sharing inter-laboratory data for research purposes, microspheres which bind monoclonal antibodies at known capacities can be used for establishing daily standard curves,13 though for single laboratory use the extra expense seems unnecessary.
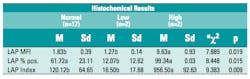
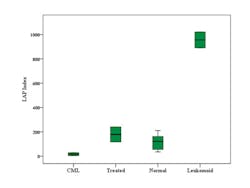
A “LAP Index” consisting of the LAP-PE MFI x % LAP-PE positive mature granulocytes, was found to be more useful than only MFI or % positive cells. (Table 1) This index reflects a similar approach to the histochemical scoring. When reporting, the local normal range can be reported, including the MFI and % positive if desired. Previous works showed overlap when using only % positive cells or MFI for normals vs. CML.5,6 This study showed that using a LAP index, CML, and leukemoid reaction could clearly be distinguished from laboratory specific established normal values (Figure 3).
Table 1. LAP Index vs LAP MFI or LAP % positive compared to histochemical results of low, normal, and high. LAP Index is a calculated value of the product of LAP median fluorescent intensity (MFI) and LAP % positive population of gated mature granulocytes. The lowest p value was found for LAP index. * Kruskal-Wallis non-parametric test
Figure 3. LAP index usefulness in differential diagnosis.
While LAP has fallen out of favor in diagnostic use, there remains a use in researching neutrophil activation status in various disease states.14-17 Patients with chronic myeloproliferative disorders (MPD) showed granulocyte activation parameters, and those with the JAK2 V617F mutation had significantly higher neutrophil counts, total white blood cell counts, and LAP levels. A significant relationship between percentage of JAK2 mutant alleles and LAP expression was found; high LAP scores can predict JAK2 mutation.16,18,19 LAP is a possible surrogate marker in MDS progression;20 which extracellular signals affect LAP levels remains to be researched fully.21
Thus, the LAP score still has clinical and diagnostic implications. In this study it was shown that the flow cytometric method is at least as reliable and more quantitative as the histochemical method in determining an LAP score. In the peripheral hospital setting LAP index is another tool used in the diagnostic pathway by physicians who do not have immediate access to molecular genetic testing.
Najib Dally, MD, is the head of the Hematology Institute at Ziv Medical Center in Safed. He also lectures for the Bar Ilan Medical Faculty and holds a position in the Institute of Hematology and Bone Marrow Transplantation at Rambam Medical Center. Adi Sharabi-Nov, MA, is the statistician at Ziv Medical Center. She also lectures at Tel Hai College in statistics. Elizabeth Eshel, BA, is coordinator for the flow cytometry unit at Ziv Medical Center. She is currently studying for a MSc in Biomedical Science.
References
- Okun DB, Tanaka KR. Leukocyte alkaline phosphatase. Am J Hematol. 1978;4(3):293-299.
- Ackerman GA. Histochemical differentiation during neutrophil development and maturation. Ann N Y Acad Sci. 1964;113:537-565.
- Hayhoe FG, Quaglino D. Cytochemical demonstration and measurement of leucocyte alkaline phosphatase activity in normal and pathological states by a modified azo-dye coupling technique. Br J Haematol. 1958;4(4):375-389.
- Material Safety Data Sheet Formalin Solution. [10/20/05; cited]; Available from: http://www.aphis.usda.gov/animal_health/lab_info_services/downloads/MSDS_Formalin.pdf. Accessed June 25, 2013.
- Rambaldi A, Masuhara K, Borleri GM, et al. Flow cytometry of leucocyte alkaline phosphatase in normal and pathologic leucocytes. Br J Haematol. 1997;96(4):815-822.
- Okamoto T, Okada M, Yamada S, et al. Flow-cytometric analysis of leukocyte alkaline phosphatase in myelodysplastic syndromes. Acta Haematol. 1999;102(2):89-93.
- Hanson EM, Agosti SJ, Kang LC. Can a clinical LAP procedure be made reproducible and easy to perform? MLO. 2009;41(11):44-46.
- Bain BJ, Bates I, Laffan MA, Lewis SM. Neutrophil alkaline phosphatase. In: Houston M, ed. Dacie and Lewis Practical Haematology. Eleventh Edition. Elsevier Inc.; 2011.
- Porwit A, McCullough J, Erber WN. Chronic myelogenous leukemia. In: Houston M, ed. Blood and Bone Marrow Pathology. Second edition: Elsevier Inc.; 2011.
- WHO. Classification of Tumours of Haematopoietic and Lymphoid Tissues. Fourth Edition. Lyon: IARC; 2008.
- Kanegae MP, Ximenes VF, Falcao RP, et al. Chemiluminescent determination of leukocyte alkaline phosphatase: an advantageous alternative to the cytochemical assay. J Clin Lab Anal. 2007;21(2):91-96.
- Wilson PD, Rustin GJ, Peters TJ. The ultrastructural localization of human neutrophil alkaline phosphatase in normal individuals during pregnancy and in patients with chronic granulocytic leukaemia. Histochem J. 1981;13(1):31-43.
- Gratama JW, D’Hautcourt JL, Mandy F, et al. European Working Group on Clinical Cell Analysis. Flow cytometric quantitation of immunofluorescence intensity: problems and perspectives. Cytometry. 1998;33(2):166-178.
- Cervantes F, Arellano-Rodrigo E, Alvarez-Larran A. Blood cell activation in myeloproliferative neoplasms. Haematologica. 2009;94(11):1484-1488.
- Falanga A, Marchetti M, Evangelista V, et al. Polymorphonuclear leukocyte activation and hemostasis in patients with essential thrombocythemia and polycythemia vera. Blood. 2000;96(13):4261-4266.
- Basquiera AL, Fassetta F, Soria N, Barral JM, Ricchi B, Garcia JJ. Accuracy of leukocyte alkaline phosphatase score to predict JAK2 V617F mutation. Haematologica. 2007;92(5):704-705.
- Rambaldi A, Terao M, Bettoni S, et al. Expression of leukocyte alkaline phosphatase gene in normal and leukemic cells: regulation of the transcript by granulocyte colony-stimulating factor. Blood. 1990;76(12):2565-2571.
- Campbell PJ, Scott LM, Buck G, et al. Definition of subtypes of essential thrombocythaemia and relation to polycythaemia vera based on JAK2 V617F mutation status: a prospective study. Lancet. 2005;366(9501):1945-1953.
- Passamonti F, Rumi E, Pietra D, et al. Relation between JAK2 (V617F) mutation status, granulocyte activation, and constitutive mobilization of CD34+ cells into peripheral blood in myeloproliferative disorders. Blood. 2006;107(9):3676-3682.
- Lipshitz J, Limaye S, Patel D. Leukocyte alkaline phosphatase score correlation with bone marrow blast percentage in myelodysplastic syndrome. Acta Haematol. 2010;124(3):179-181.
- Shanmugham LN, Petrarca C, Castellani ML, et al. IL-1beta induces alkaline phosphatase in human phagocytes. Arch Med Res. 2007;38(1):39-44.