News
Disaster preparedness
Power-protection course. On January 24, 2004, Franek Technologies offers a course for laboratory managers Power Protection of Mission Critical Medical and Laboratory Equipment on laboratory disaster preparedness. The course will be held at the University of California-Irvines Learning Center in Orange, CA, Room 212. Registration information is available at http://unex.uci.edu/catalog/cart.asp (enter registration number 00259 into the course catalog form), or call (949) 824-5414.States to be graded on bioterror plans. To test each state on its readiness for bioterrorism and health emergencies, a scoring system was implemented by the Centers for Disease Control and Prevention (CDC) this month. How quickly a smallpox vaccination clinic could be operational is one area that will be evaluated. According to Joe Henderson head of the CDCs bioterrorism-preparedness effort in the event of a smallpox outbreak, it could take three days to verify the diagnosis. Thus, states must be prepared to vaccinate all their residents within 10 days if the deadly virus were to be unleashed during a terrorist attack. He commended Floridas example for storing its vaccine allotment in every county for faster local access. Other programs will be evaluated by an independent group hired by the CDC and include ways to detect early warning signs of disease, track outbreaks, train doctors, and communicate with the public. The actual testing may begin early summer. Experimental Ebola vaccine given. An experimental vaccine designed to protect against Ebola is filled with the biological essence of one of the worlds deadliest diseases, which rapidly kills 50% to 90% of its victims. Like the smallpox virus and anthrax bacterium, Ebola virus is a potential bioterrorism agent. An aerosolized form would be even more deadly.
This first Ebola vaccine was made without a single component from the virus itself. Researchers at Vical a biotechnology company in California made the laboratory-synthesized strands of DNA mimic those found in Ebola. Key components of the virus were removed including those that trigger the illness, and that which might allow the DNA to recombine with another virus DNA to form a new and potentially disease-causing bug. The product is spray-blasted into a patients arm, and was designed to rally the immune system without causing any symptoms of the disease itself. The DNA enters subcutaneous skin cells, which use it to make Ebola proteins. Immune-system cells attack those proteins and then are primed forever to fight a real Ebola infection. The National Institutes of Healths plan is to follow the first DNA shot, given to a volunteer nurse there, with a booster made of an adenovirus engineered to contain Ebola DNA. Blood tests will track his and other volunteers immune-system responses for a year. Much larger human studies will eventually be conducted to provide final proof that the vaccine is safe for large populations.
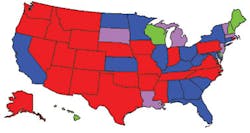
Weekly influenza activity estimates reported by state and territorial epidemiologistsWeek ending December 6, 2003 Week 49
Source: CDC at www.cdc.gov/flu/weekly
2004: Vol. 36, No. 1©
2004 Nelson Publishing, Inc. All rights reserved.