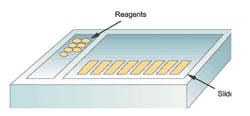
Figure 1. Platform configuration: matrix/array-style stainers
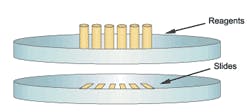
Figure 2. Platform configuration: rotary/carousel-style stainers
The following review is intended to educate the scientific community as a whole and the buying public, in particular, on various aspects of the automated slide-staining systems designed for histochemistry or special stains (SS) as they are commonly known immunohistochemistry (IHC), and in situ hybridization (ISH). With so many instruments on the market, this review should prove useful to individuals who are currently shopping for such a system, and assist them in making better-informed acquisition decisions.
First, it is important to note that automated slide-staining systems used for SS, IHC, and ISH procedures are fundamentally different from instruments designed for routine H&E (hematoxylin and eosin) staining, in that reagents are applied to
individual slides in small, controlled volumes rather than fully immersing slides in a large volume of reagent. This is necessary because the chemical reactions involved in nonroutine staining protocols are far more sensitive, and often require that a series of reagents be applied in a specified order to create bound molecular complexes. For simplicitys sake, these instruments will be referred to as special stainers throughout the remainder of this article.
At the present time, the vast majority of special stainers installed in this country are used to perform only IHC procedures. A smaller percentage is used for SS procedures and even fewer are used for ISH. The main reason that such systems are not used more often for special stains is that they do not always provide adequate staining consistency. This shortcoming can be better explained by way of examining one of the inherent advantages of manual staining.
While performing a routine SS procedure, slides are usually stained as a batch, in a vessel like a Coplin jar and when the incubation period for a given reagent has ended, this reagent is poured out and replaced with a rinse solution or another reagent in this way, the entire group of slides can be treated simultaneously. This simultaneous processing is simply not possible with an automated stainer, which takes considerable time to move from slide to slide to perform the necessary rinses. And the main reason that more special stainers are not used for ISH is that the demand for these procedures is still not that great within clinical histology labs. In addition, very few of the available special stainers provide a means to heat slides, which many scientists believe is necessary to adequately perform the denaturation and hybridization steps required during ISH procedures.
Different shapes and sizes
One of the most noticeable features of special stainers is their physical configuration, or format, as well as slide and reagent capacities. Some units resemble an array or matrix, since slides are placed on removable support racks arranged in rows and columns (Figure 1), while the alternative format could be described as a carousel or rotary in nature, because slides (and reagent containers) are arranged in a circular pattern on a platter-like support (Figure 2). One of the major
advantages of array-format stainers is that slides can easily be removed as staining procedures are completed, and, in the case of some models, may even allow the operator to add new slides to a run in progress, a feature that is commonly referred to as continuous access. By comparison, one of the major disadvantages of carousel-format stainers (Figure 2) is that no slides can be removed until all procedures have been completed. This could have productivity implications in a busy lab, since slides with relatively short protocols must remain on the stainer until the longer protocols have ended. A summary of the main technological differences between special stainers is presented in Table 1.
Different operating modes
One of the most important aspects of special stainers that requires further explanation is whether a system is closed or open, which has both conceptual and physical implications.
Conceptually speaking, closed systems take their name from the fact that they usually restrict the operators ability to use reagents obtained from sources other than the instruments distributor, and often limit the operators ability to modify staining protocols. In addition, such instruments can be considered
physically closed because they require that slides be placed on a platter that becomes inaccessible during the staining procedure. In other words, no slides can be removed from the platform until all protocols have been completed. Closed systems also include those that automatically determine which staining protocol to apply to slides by reading a barcode label that is applied prior to starting the run. Not only do such units employ barcode-labeled slides, but they also require use of pre-filled, barcode-labeled reagent containers, which imposes financial disincentives for using products obtained from other vendors.
Table 1. Summary of technological differences between special stainers
| Slide Placement Format | Operating Modes and Notable Requirements | Reagent Application Methods |
Advantages and Disadvantages |
| Matrix / Array Slides arranged in rows on removable, accessible racks Slides can be removed at any time | Usually Open Requires operator to select protocols and place slides on stainer, then instructs operator where to place reagents Allows use of reagents obtained from a variety of sources Allows greater ability to modify staining protocols |
Disposable Pipette Tips and Reusable/Disposable Reagent Vials |
OR
Reusable (Rinsed) Probes and Reusable/Dispos-able Reagent Vials
Typically larger capacity than Carousel/ Rotary-format stainers
Typically shorter procedures (runs) than Carousel/Rotary
Pipette tip-type systems eliminate reagent carry-over (contamination) and allow for more accurate/efficient reagent usage
Reusable Probe-type systems may result in reagent carry-over if inadequately rinsed Carousel / Rotary
Slides arranged in a circular fashion on an inaccessible platter
Slides can only be removed at end of runUsually Closed
Automatically determines appropriate staining protocol through barcoded slide labels
More restrictive in the use of reagents obtained from non-vendor sources
More restrictive in the ability to modify staining protocols
Disposable (syringe-like) Dispensers
OR
Disposable (printer cartridge-like) Dispensers
Typically smaller capacity than Matrix/Array-format stainers
Typically longer procedures (runs) than Matrix/Array
Neither reagent application method is as accurate/efficient as those used in Matrix/Array-format stainers; for example, dispensers only allow for a fixed-volume (e.g. 100uL) application of all reagents
*SS = Special Stains
So-called open systems, on the other hand, are conceptually open in that they are designed to allow use of reagents obtained from sources other than the instruments distributor, and
physically open because slides are placed on an accessible platform during staining procedures, which can be opened while in operation. When operating this type of stainer, the user must first select the desired staining protocol to be applied to each slide, and then the system will instruct the operator where to place the reagent vials. Open systems nearly always allow the operator to modify various parameters within staining protocols, and some even allow the operator to create his own protocols, rather than require use of those provided by the vendor.
Another technologically significant aspect of these special stainers is the means by which reagents are applied to slides. Although this may seem unimportant to the casual observer, it should be noted that each reagent-delivery technique has its inherent weaknesses. The most commonly used method involves a probe-like device, similar to those used in chemistry analyzers. Probe-type systems are only found on array-format stainers, and must be rinsed thoroughly between different reagents in order to prevent carry-over, since contamination of one reagent with another could result in false-positive staining. An alternative reagent delivery method mimics the steps involved in manual slide staining procedures through use of disposable pipette tips, which are loaded and ejected by a robotic mechanism. Pipettor-style reagent dispensing is only found on array-format stainers, and if the force with which the tips are picked up is not properly calibrated, the instrument may drop tips during the staining procedure. Finally, there are carousel-style instruments that deliver reagents via spring-loaded, syringe-like or printer cartridge-like dispensers. In these systems, once the appropriate reagent container has been rotated into position above the appropriate slide, a mechanism within the instrument releases a pre-set volume of reagent. Some of the inherent shortcomings of this method are that the reagent containers are not designed to deliver a precise volume of reagent (like pipette tips and probes do), which may result in the waste/loss of costly reagents. A summary of the basic features of special stainers is presented in Table 2.
Table 2. Commercially available systems Basic features
| Vendor | Web Address | Product Name |
Slide Placement Format |
Operating Modes |
Intended Staining Capabilities* |
Reagent Application Method |
| BioCare Medical | www.biocare.net | Nemesis 3600 | Matrix/Array |
Barcode & Open |
IHC, SS |
Rinsed Probe |
| BioGenex Laboratories | www.biogenex.com | i6000 | Matrix/Array |
Barcode & Open |
IHC, SS, ISH |
Pipette Tips |
| DakoCytomation | www.dakocytomation.com | Autostainer Plus |
Matrix/Array |
Barcode & Open |
IHC |
Rinsed Probe |
| DakoCytomation | www.dakocytomation.com | Artisan |
Carousel/Rotary |
Barcode pen |
IHC, SS |
Disposable Dispenser |
| Lab Vision-Neomarkers | www.labvision.com | Autostainer 360-2D |
Matrix/Array |
Open |
IHC |
Rinsed Probe |
| Lab Vision-Neomarkers | www.labvision.com | Autostainer 720-2D |
Matrix/Array |
Open |
IHC |
Rinsed Probe |
| Ventana Med. Systems | www.ventanamed.com | Benchmark XT | Carousel/Rotary |
Barcode |
IHC, SS, ISH |
Disposable Dispenser |
| Ventana Med. Systems | www.ventanamed.com | Benchmark |
Carousel/Rotary |
Barcode |
IHC, SS, ISH |
Disposable Dispenser |
| Ventana Med. Systems | www.ventanamed.com | NexES |
Carousel/Rotary |
Barcode |
IHC, SS |
Disposable Dispenser |
| Vision Biosystems | www.vision-bio.com | Bond-maX |
Matrix/Array | Barcode & Open |
IHC, ISH |
Rinsed Probe |
| Vision Biosystems | www.vision-bio.com | Bond-X |
Matrix/Array | Barcode & Open |
IHC |
Rinsed Probe |
| Zymed Laboratories | www.zymed.com | Mozaic |
Matrix/Array | Open |
IHC, ISH |
Rinsed Probe |
Table 3. Commercially available systems Special features
| Vendor | ProductName | Slide Capacity | Reagent Capacity |
Barcoded Slide Labels* | Barcoded Reagent Containers** |
Continuous Processing | Slide Heating | Variable Dispense Volume |
Waste Separation |
| BioCare Medical | Nemesis 3600 | 36 | 40 | Yes | Yes | Yes | No | Yes | Yes |
| BioGenex Laboratories | i6000 | 60 | 60 | Yes | Yes | Yes | No | Yes | No |
| DakoCytomation | Autostainer Plus | 48 | 64 | Yes | No | No | No | Yes | Yes |
| DakoCytomation | Artisan | 48 | 50 | Yes | No | No | Yes | No | Yes |
| Lab Vision-Neomarkers | Autostainer 360-2D | 36 | 40 | Yes | Yes | Yes | No | Yes | Yes |
| Lab Vision-Neomarkers | Autostainer 720-2D | 72 | 84 | Yes | Yes | Yes | No | Yes | Yes |
| Ventana Med. Systems | Benchmark XT | 30 | 35 | Yes | Yes | No | Yes | No | No |
| Ventana Med. Systems | Benchmark | 20 | 25 | Yes | Yes | No | Yes | No | No |
| Ventana Med. Systems | NexES | 20 | 25 | Yes | Yes | No | Yes | No | No |
| Vision Biosystems | Bond-maX | 30 | 36 | Yes | Yes | Yes | Yes | No | No |
| Vision Biosystems | Bond-X | 30 | 36 | Yes | Yes | Yes | Yes | No | No |
| Zymed Laboratories | Mozaic | 96 | 54 | No | No | No | No | Yes | No |
*Usually permits automatic protocol entry
**Usually permits volume tracking
Special requirements
Finally, special note should be made of the unique features of some of these slide-staining systems. For example, Vision Biosystems and Zymed Laboratories units require that a disposable plastic plate be placed over each slide when loaded. These covertiles, as one vendor calls them, are designed to ensure that reagent solutions spread evenly over the slide, and remain in place during the ensuing incubation period. Another unconventional technique is employed on Ventana Medical Systems units: After a fixed volume of staining reagent is dispensed onto a slide, it is dropped immediately, followed by a solution known as liquid cover slip, which helps the staining reagent spread evenly over the slide. A summary of other important slide stainer features is presented in Table 3.
Everyones needs are different
Individuals interested in evaluating slide stainers for the first time are normally concerned with the following issues: 1) ability to use reagents from different vendors; 2) the systems slide and reagent capacity; 3) ability of each vendor to provide them with optimized reagents for the desired application(s); and 4) the amount of hands-on time required of the operator (for slide loading/unloading and protocol selection/modification). On the other hand, experienced users and those with more advanced needs are usually more concerned with: 1) the systems format (i.e. array vs. carousel); 2) the ability of the instrument to apply heat to individual slides within a narrow temperature range; 3) the reagent application method; and 4) the degree to which essential staining parameters (such as incubation time and/or reagent dispense volume) can be modified.
It is important to point out several of the less tangible aspects involved in the process of evaluating special stainers. These include considerations such as a systems overall throughput, the ability to run multiple units with one computer, and a vendors ability to provide a large and varied enough selection of pre-packaged, optimized reagents to meet the labs staining needs, as well appropriate training, troubleshooting, technical support, and field service.
Joe Myers is a clinical laboratory scientist and sales professional who resides in Clearwater, FL. He has extensive experience in the use of immunohistochemistry, in situ hybridization, and a variety of automated instruments. Joe can be reached at
[email protected].
©
2004 Nelson Publishing, Inc. All rights reserved.