Screening tests for coagulation defects can be run in most clinical laboratories. Once a defect is discovered, it is often necessary to have a specialized coagulation laboratory perform nonroutine tests to determine the specific defect. This article discusses some of these less common tests.
Background
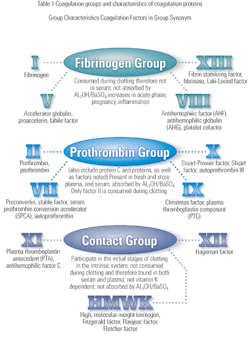
Hemostasis describes a complex process through which bleeding stops spontaneously following injury, and blood is maintained in the liquid state. The process involves both cellular and biochemical events that function together to keep blood in the liquid state within the vascular system. During the process of hemostasis, a thrombus is formed, and blood flow is re-established during the healing
process.1, 2 A complete balance of the bodys tendency towards clotting and bleeding is maintained. Hemostasis is achieved through the interaction of several systems, which include the vascular system, the coagulation system, the fibrinolytic system and platelets, as well as the kinin system, serine protease inhibitors and the complement system.3,4 The different systems work together when the blood vessel endothelial linings are disrupted by mechanical trauma, physical agents or chemical trauma. The fibrinolytic process is involved with the dissolution of clots, which are produced to stop bleeding. Consequently, there is a delicate balance between the production and dissolution of clot during the hemostatic process. When this balance in the hemostatic system is deranged, thrombosis or hemorrhage may result due to hypercoagulation or hypocoagulation respectively.1,4
Hemostasis consists of two components referred to as primary and secondary hemostasis. Primary hemostasis involves the adhesion of platelets to the injured vessels. The primary response is mediated by the platelet membrane glycoprotein Ib and von Willebrand factor (vWF).2 The interaction between platelets and the vascular endothelium results in a series of reactions, which culminate in the formation of the thrombus. Secondary hemostasis involves the response of the coagulation process to injury through the activation of coagulation proteins, leading to the formation of the fibrin clot.4
Most clinical laboratories are capable of performing the first line of screening tests to determine whether there is a coagulation defect and to roughly classify its type. It is necessary, however, to refer the problem to a specialized coagulation laboratory for the less common tests. This is because special reagent systems are necessary, or because people with specialized expertise and experience must run the tests.
Specialized tests for coagulation factors
The one-stage quantitative assay for factors VIII, IX, XI and XII. The APTT is the basis of this test system. It is based on the ability of patient plasma to correct specific factor-deficient plasma, and the results in percent activity are obtained from an activity curve.2 An ELISA technique has been developed to measure factor VIII antibodies in hemophilia patients. The assay utilizes binding of the antibodies in the plasma to solid phase antigen, which is subsequently detected by a human polyclonal IgG labeled with the alkaline phosphatase-p-nitrophenyl phosphate substrate system.5
Test for fibrin formation
Fibrinogen assay. Fibrinogen assay is used to measure factor concentration in plasma. Fibrinogen has been identified as an independent risk factor for cardiovascular disease and is associated with traditional cardiovascular risk factors. Also, the role of elevated fibrinogen in thrombosis suggests that it may be on the causal pathway for certain risk factors to exert their effect. These associations remain incompletely characterized. Moreover, the optimal fibrinogen assay for risk stratification is uncertain.6
The coagulation status of infant and pediatric patients can be severely compromised during the course of cardiopulmonary bypass due primarily to hemodilution and hypothermia. Fibrinogen level is one source of information necessary to assess the coagulation status of a patient. An accurate and expedient method to determine the fibrinogen level would allow for earlier initiation of coagulation therapy to prevent excessive postoperative bleeding.7
Fibrinogen can be quantitated by various methods including precipitation or denaturation methods, turbidimetric or fibrin clot density method, coagulable protein assays, as well as immunologic assays, which utilize antibodies to fibrinogen in assays, such as radial immunodiffusion, measurement of turbidity or rocket immunoelectrophoresis and the modified thrombin clotting time, which is the most widely performed clinical fibrinogen assay.4 The clotting time of diluted plasma to which a high concentration of thrombin has been added is inversely proportional to the fibrinogen concentration. Quantitation is achieved through the use of standards with known concentrations of fibrinogen.
Thrombin time
The thrombin time is the time needed for thrombin to convert fibrinogen to an insoluble fibrin clot. It is triggered by the addition of thrombin to the sample and thus bypasses prior steps in the coagulation
cascade.2 The test does not measure defects in the intrinsic or extrinsic pathways. It is affected by abnormal fibrinogen, dysfibrinogenemia and the presence of circulating anticoagulants including heparin and FDPs.2 Surgery induces immediate hypercoagulability by direct alteration of the vascular bed, release of procoagulant substances from the extravascular spaces and blood flow decrease, and delayed hypercoagulation in response to tissue damage, which triggers inflammatory responses. Thus, the postoperative period represents a high-risk time for thrombosis. Recognition of high-risk individuals would make it possible to improve thromboembolism prevention.9 Platelet-induced thrombin generation time is a newly developed global coagulation assay in which a small amount of partially anticoagulated platelet-rich plasma is rotated in a disc-shaped cuvette within the light beam of a photometer. The time intervals from onset of rotation until aggregation and coagulation of the sample are registered.10
Reptilase time
This test is similar to the thrombin time; however, the clotting sequence is initiated with the snake venom enzyme, reptilase. The enzyme is thrombin-like in nature and hydrolyzes fibrinopeptide A from the intact fibrinogen molecule, unlike thrombin, which hydrolyzes fibrinopetide A and B from fibrinogen.2 The clot that is formed is fragile compared to that formed with thrombin. Reptilase is not inhibited by heparin, and the effect of FDPs on reptilase is minimal.2 Reptilase test is used for screening dysfibrinogenemia.
Dysfibrinogenemia is a coagulation disorder caused by a variety of structural abnormalities in the fibrinogen molecule that result in abnormal fibrinogen function. It can be inherited or acquired. The inherited form is associated with increased risk of bleeding, thrombosis or both in the same patient or family. Traditionally, dysfibrinogenemia is diagnosed by abnormal tests of fibrin clot formation; the thrombin time and reptilase time are the screening tests, and the fibrinogen clotting activity-antigen ratio is the confirmatory test. The inherited form is diagnosed by demonstrating similar laboratory test abnormalities in family members and, if necessary, by analysis of the fibrinogen protein or fibrinogen genes in the patient. The acquired form is diagnosed by demonstrating abnormal liver function tests and by ruling out dysfibrinogenemia in family members.11
Test for von Willebrand disease
For more than two decades, the ristocetin cofactor (RCo) assay, which measures the vWF-mediated agglutination of platelets in the presence of the antibiotic ristocetin, has been the most common method for measuring the functional activity of vWF.12 There is, however, general agreement among clinical analysts that this method has major practical disadvantages in performance and reproducibility.
Today, collagen-binding assays based on the ELISA technique that measure the interaction of vWF and collagen are an alternative analytic procedure based on a more physiological function than that of the RCo procedure.12 Circulating plasma vWF antigen is a marker of generalized endothelial dysfunction and
atherothrombosis.13 The vWF antigen can be quantitated using ELISA, Laurell rocket electrophoresis or latex immunoassay. The collagen-binding assay was recently recommended as the new method for determining vWF activity. The assay is based on measurement of the quantity of vWF molecules bound to collagen, similar to the procedure for ELISA.14 The factor is quantified, regardless of its functionality. A most common technique is EIA employing the sandwich method. A microtiter plate is coated with specific rabbit antihuman vWF, and the antibody captures the vWF to be measured. Rabbit antivWF antibody coupled with peroxidase binds to the remaining free antigenic determinants of vWF, forming the sandwich. The bound enzyme is then detected by its activities on the substrate orthophenylenediamine in the presence of hydrogen peroxide.2 The intensity of color produced is directly proportional to the vWF concentration in the plasma sample.
Regulatory protein assays
Antithrombin-III. Antithrombin-III (AT-III) is a natural occurring inhibitor of blood coagulation and plays an important part in maintaining blood in the fluid state. Antithrombin-III is synthesized in the liver and circulates in the plasma. It is responsible for the neutralization of the activity of thrombin, factors IXa, Xa, XIa, and XIIa, as well as plasmin. The inhibition of thrombin by AT-III is greatly accelerated by
heparin.2 Antithrombin-III assays are performed to assess response to heparin therapy and efficacy of antithrombin-III concentrate therapy and in diagnosing hereditary thrombophilia, deep venous thrombosis, pulmonary embolus and DIC. Synthetic substrate assays for antithrombin-III are the methods of choice; however, most existing assay systems are semiautomated.15 Hereditary deficiency of AT-III is a well-established cause of recurrent venous thrombosis. Cross-reactivity of heparin cofactor II in assays of AT-III may, in some cases, interfere with the ability to diagnose hereditary deficiency of AT-III.16
Protein C. Protein C is a vitamin K-dependent serine protease that functions as a major regulatory protein in the control of coagulation.2 It is a potent anticoagulant that inactivates factors Va and VIIIa and also enhances fibrinolytic activity in plasma. A deficiency of protein C is a risk factor in thromboembolic disease. Activated protein C resistance results from a mutation in the factor V gene, known as factor V Leiden, and makes the factor V molecule resistant to proteolytic activity of activated protein C. The diagnosis of protein C deficiency is determined by immunologic and functional assays. Immunological methods for protein C measurement involve the Laurell rocket immunoelectrophoresis technique, radioimmunoassay technique or ELISA.
Protein S. Protein S functions as a cofactor for protein C and may also be measured immunologically or functionally. Protein S in circulation is in a dynamic equilibrium with C4b binding protein (C4bBP), thus affecting the measurement of free protein S antigen.17 Tsuda, et al,17 examined the issue of overestimation of the free protein S concentration with current immunoassays due to the dynamic equilibrium and proposed a new method for its accurate determination.
They tested their assay system at different reaction temperatures using purified free protein S, protein S-C4bBP complexes, plasma samples and a commercially available free protein S assay kit. They found that at a reaction temperature of 37oC, the free protein S fraction increased from 0.5 ng/mL (at 4oC) to 7.8 ng/mL, and from 4.5 ng/mL (at 4oC) to 56 ng/mL when the concentration of the assayed protein S-C4bBP complexes was 20 ng/mL and 200 ng/mL, respectively. In plasma samples, free protein S levels were approximately 0.8 mg/mL and 6 pg/mL higher at 25oC and 37oC, respectively compared to measurements at 4oC.
They concluded that measurements of free protein S in plasma using a commercially available assay kit were approximately 0.6 mg/mL higher at 25oC than measurements performed at 4oC. Dynamic equilibrium between protein S and C4bBP affects the measurement of free protein S antigen. Measurement of free protein S antigen should be performed under conditions where protein S is not dissociated from protein S-C4bBP complexes, as exemplified by assay at low temperature.
Tests for fibrinolytic pathway
The fibrinolytic pathway is involved with clot dissolution following the release of plasmin from plasminogen. Samples for fibrinolytic system components should be obtained at standardized time, preferably early morning following an overnight fast and at least 15-minute rest.4 The sample should be processed immediately after collection, centrifuged to be platelet-free and if not tested immediately, placed in plastic vials and frozen at 70oC.
Plasminogen assay
Plasminogen should be assayed by both the immunologic and functional assays in order to detect nonfunctional molecules. Immunologic assays by radial immunodiffusion may be employed. Patients plasma is added into a well cut into an agarose matrix containing plasminogen antibody. The plasma is allowed to diffuse from the well, and the interaction of patients plasminogen with antibody results in an immunoprecipitation reaction. The diameter is measured and compared to that caused by a control plasma. The test takes about 48 hours to complete.4 In the functional assay, an excess of plasminogen activator, such as streptokinase, is added to a plasma sample. The resultant plasminogen-streptokinase complex generates plasmin activity that reacts with a synthetic chromogenic substrate. This produces a color change that is proportional to the plasminogen level in the plasma.4
Tissue plasminogen activators
The level of tissue plasminogen activators (TPA) may be determined using enzyme linked immunosorbent assay sandwich technique. The test sample is added to a microtiter well coated with antiTPA antibodies, which are labeled with peroxidase. The wells are washed to remove unbound conjugate after which a color-linked peroxidase substrate is added. The amount of yellow color produced is directly proportional to the amount of TPA present in the sample.4
Plasmin activator inhibitor-1
Endothelial cells, hepatocytes and megakaryocytes synthesize plasminogen activator inhibitor-1 (PAI). Increased levels of PAI are associated with thrombotic disorders.4 A two-stage enzyme assay is available for the measurement of PAI. A standard amount of TPA is added to a plasma sample and incubated for a specific time and the TPA complexes with the PAI. Plasmin inhibitors are removed by acidification, and the unbound TPA is quantified by adding the sample to a mixture of PAI-deficient plasma containing plasminogen and a chromogenic substrate. The color change produced is measured and the amount of color produced is inversely proportional to the amount of PAI.4
Conclusion
Clinical examination provides useful information that may lead to the diagnosis of hemostatic disorders. An accurate diagnosis of the hemostatic disease ultimately depends, however, on laboratory testing. Various laboratory tests are available for the diagnosis of problems associated with the hemostasis. Many of these tests are specific for different components of the hemostatic system and require the expertise of a coagulation laboratory that sees a high volume of coagulation problems. The clinical laboratory will continue to play a very important role in the diagnosis and treatment of hemostatic diseases. An understanding of those tests that must be referred is essential to appropriate referral of the tests.
Henry Ogedegbe, PhD, BB(ASCP), C(ASCP)SC, CLS(NCA), NRCCCC, and Halcyon St. Hill, EdD, MS, MT(ASCP), CLS(NCA), are affiliated with the College of Health Professions, Florida Gulf Coast University, Fort Myers, FL.
References
- Rodak BF. Hematology, Clinical Principles and Applications. 2nd ed. Philadelphia: W.B Saunders Company; 2002:609-753.
- Harmening DM. Clinical Hematology and Fundamentals of Hemostasis.
4th ed. Philadelphia: F. D. Davis Company; 2002 471-494 . - Hoffmeister HM. Overview of the relevant aspects of the blood coagulation systemfocus and cardiovascular hemostasis. Kongressbd Dtsch Ges Chir Kongr. 2001;118:572-575.
- Stiene-Martin EA, Lotspeich-Steininger CA, Koepke JA. Clinical Hematology. Principles, Procedures, Correlations. 2nd ed. Philadelphia: Lippincott; 1998 599-611.
- Shetty S, Ghosh K, Mohanty D. An ELISA Assay for the Detection of Factor VIII Antibodies – Comparison with the Conventional Bethesda Assay in a Large Cohort ofHaemophilia Samples. Acta Haematol. 2003;109(1):18-22
- Stec JJ, Silbershatz H, Tofler GH, Matheney TH, Sutherland P, Lipinska I, Massaro JM, Wilson PF, Muller JE, DAgostino RB Sr. Association of fibrinogen with cardiovascular risk factors and cardiovascular disease in the Framingham Offspring Population. Circulation. 2000;102(14):1634-1638.
- Matthews DR, Ecklund JM, Hennein H. Clinical comparison of patient-side fibrinogen assay and common laboratory analyzer in pediatric cardiopulmonary bypass. J Extra Corpor Technol .1995;27(3):126-131.
- Ferreira CN, Vieira LM, Dusse LM, Reis CV, Amaral CF, Esteves WA, Fenelon LM, Carvalho MG. Evaluation of the blood coagulation mechanism and platelet aggregation in individuals with mechanical or biological heart prostheses. Blood Coagul Fibrinolysis. 2002;13(2):129-134.
- Freyburger G, Dubreuil M, Audebert A, Labrouche S, Pistre JC, Molinari I, Dubecq F, Laville C, Villanove X. Changes in haemostasis after laparoscopic surgery in gynaecology: contribution of the thrombin generation test. Haemostasis. 2001;31(1):32-41.
- Radziwon P, Boczkowska-Radziwon B, Schenk JF, Wojtukiewicz MZ, Kloczko J, Giedrojc J, Breddin HK. Platelet activation and its role in thrombin generation in platelet-induced thrombin generation time. Thromb Res. 2000;100(5):419-26.
- Cunningham MT, Brandt JT, Laposata M, Olson JD. Laboratory diagnosis of dysfibrinogenemia. Arch Pathol Lab Med. 2002;126(4):499-505.
- Turecek PL, Siekmann J, Schwarz HP. Comparative study on collagen-binding enzyme-linked immunosorbent assay and ristocetin cofactor activity assays for detection of functional activity of von Willebrand factor. Semin Thromb Hemost. 2002;28(2):149-160.
- Rabbani LE, Seminario NA, Sciacca RR, Chen HJ, Giardina EG. Oral conjugated equine estrogen increases plasma von Willebrand factor in postmenopausal women. J Am Coll Cardiol. 2002;40(11):1991-1999.
- Paczuski R. Determination of von Willebrand factor activity with collagen-binding assay and diagnosis of von Willebrand disease: effect of collagen source and coating conditions. J Lab Clin Med. 2002;140(4):250-254.
- Bick RL, Wheeler A. Fully automated antithrombin-III assays by synthetic substrate on the Multistat III. Am J Clin Pathol. 1987;88(2):192-197.
- Hortin GL, Tollefsen DM, Santoro SAAssessment of interference by heparin cofactor II in the DuPont aca antithrombin-III assay. Am J Clin Pathol. 1988;89(4):515-517.
- Tsuda T, Tsuda H, Yoshimura H, Hamasaki N. Dynamic equilibrium between protein S and C4b binding protein is important for accurate determination of free protein S antigen. Clin Chem Lab Med. 2002;40(6):563-567.
© 2003 Nelson Publishing, Inc. All rights reserved.