The point…of point-of-care testing
The first ‘laboratory test’ was point-of-care! Physicians in ancient Egypt, Greece, and even in neolithic cultures of Amazonia, looked at a patient’s presentation and reasoned that if something inside was wrong, ‘whatever came out’ might help evaluate the cause and suggest a cure. Urine, stool, sweat, blood, etc. — look, smell, taste — they began to figure things out at the bedside.
Even in the early scientific age (18th & 19th centuries) this was true. It was only in the mid-20th century that clinical medicine became so diverse and analytically high tech that only a central or reference laboratory could manage the increasing demand for clinical testing and analysis. Today ‘s bio-engineered analytical systems, as well as data processing innovation, has allowed us to get back to the original near-patient testing for basic chemistry and cell counts as well as presumptive microbiology. Much of the complexity of routine central lab testing has been simplified such that non-specialists can use measurement systems to measure what took a specialist to perform in the recent past.
POCT versus CCT: While critical care testing (CCT) in the emergency room or intensive care unit is frequently thought of as POCT, we believe that CCT is a singular category in itself — one that typically combines in situ POCT, and central and specialty lab operations geared to most urgent priority testing.1,2,6,8,9,11,16
There certainly are commonalities between de facto POCT and CCT — primarily provision of correct testing results in a brief time interval. In order for POCT to be a requirement for an institution instead of simply an extension of CCT, one must evaluate the 1) size and scope of the institution (e.g., hospital and clinics, hospital alone, clinics alone); 2) scenarios unique to certain patient populations served; and 3) location relative to other healthcare institutions (urban, rural, remote).
POCT In large healthcare facilities/institutions (LHCF): LHCFs, especially those with campus-like configurations and both hospitalized patients and clinics, are candidates for POC testing. Laboratory leadership, as well as clinical chemists, laboratory technologists, and other analytical and technical specialists must have a seat on institutional planning committees, in addition to medical staff and institutional administration/ownership. A needs assessment must include long-range planning as well as a working philosophy of rapid adaptation to innovative technology and clinical discoveries. Such flexibility can only happen within a broad-based institutional group that has a continuing and real, rather than just pro forma presence in the institution.
For POCT, planners must consider various options for testing efficiencies particularly for care areas with the following characteristics: 1) physically remote from the central laboratory, 2) have patients with either ambulatory or retention difficulties (drug treatment clinics), 3) treat immunocompromised patients (transplant department or HIV-AIDS clinic), 4) OB/GYN clinics. Clinical providers may be concerned not only about the timeliness of test results but also about exposing their patients to individuals with other illnesses. There may also be concerns about the physical or emotional stress of traversing the institution to get lab tests performed and returning for treatment. The central laboratory may be concerned with random interjections of these patients and specimens as ‘stats’ that interrupt their efficient workflow. In each of these situations, POCT in the clinic is beneficial for clinical care, patient convenience, patient retention, and timely follow-up with the patient.
Under the general aegis of a cross-functional institutional laboratory committee as required under various regulatory bodies, laboratorians must collaborate with clinical staff in the assessment, implementation, and ongoing operation of such laboratories, particularly including the choice, use, and suitability of any information management systems. Specific needs assessment, analytical instrument selection, as well as ongoing central laboratory oversight are essential for success to ensure both the best immediate clinical care but also records/financials management. (A small reminder here for IT and billing departments — Medicare. Let us make sure the bill and list of services performed is understandable to the patient, too!)
Instrumentation: Specific solutions are dependent on institutional size and physical layout as well as geographical area covered by the institution and so, a description of options goes well beyond the scope of this report. One question to answer might be, do we need a POCT lab or just a phlebotomist and pneumatic tube connection to the central lab? One example of out-of-the-box thinking regarding the types of options to consider for a POCT chemistry system from our own experience with blood gas analyzers (BGAs): If your POCT is not in a pulmonary clinic, why would you even think of a BGA? They are for testing arterial blood and used in the emergency room.
However, did you know that some BGA configurations include sodium/potassium/bicarbonate/ TCO2/glucose/urea (BUN)/creatinine. And the specimen can be venous or arterial blood (one small vacuum tube) without centrifugation. Results are available before the patient rolls their sleeve back after the venipuncture! Little bench space used, and an MD, RN, PA, or a medical assistant (regulations permitting, of course) can do the test. Easy specimen processing, lightning-fast results, no pneumatic tube system, and no central lab (except for oversight). That’s truly point of care!
Information technology: IT is the link between and among these care areas. While the primary focus must be to provide the clinically necessary information to the caregiver accurately and in a timely fashion, setting up the systems to deliver this requires dedicated laboratory/caregiver/IT interaction as described in earlier Medical Laboratory Observer articles.13,15 For optimization of clinical utility, one must go beyond simply allowing the electronic health record (EHR) of one location to be able to ‘shake-hands.’ Electronics should enable correct rearrangement of information to ease the care being made in each clinical area without corrupting the original source of information at any of the connected locations.
Specimen characteristics: Test results, especially from systems that are multiplexed, need special attention regarding identification of specimen characteristics (i.e., anatomic source, type of specimen, and measurement basis). We would recommend more consistent and widespread use of IFCC (International Federation of Clinical Chemistry and Laboratory Medicine) specimen characteristic symbols (SCS), specifically the inclusion of these characteristics as part of the measurand name as IFCC recommends.12,15 This information is more important than ever due to the capabilities of multiplexed systems, which report values on single specimens with concentrations based on different volumes within the whole specimen.
One example of this is the blood gas systems that report on the same specimen, total hemoglobin (Hb concentration in volume of whole blood), carboxyhemoglobin (as fraction of total Hb), bicarbonate (concentration in plasma volume), and sodium (as concentration in plasma or in the plasma-water fraction [this last example being a 7% bias]). While the laboratorian may know the difference, the caregiver and especially the IT professional may not know or be aware of the significance of the difference. With the advent of more sophisticated testing methods as well as potential for various tissues being subjected to laboratory testing, provisions for more specific characterization of each measurand reported result must be made.
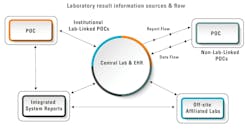
Extra institutional testing: Patients receive care in varied settings such as primary care provider/physician offices, urgent care facilities, or a combination of the two independent entities that refer patients to LHCFs. Subsequently, if testing is needed, laboratories will collect new specimens and do additional and frequently duplicative testing, adding costs and patient inconvenience. Both laboratory management and administration of LHCFs must address facilitation of the incorporation of prior testing information originating in physician offices and urgent care facilities, concomitantly assuring both compliance with legal and regulatory guidelines and simultaneously working to ensure that these results are readily available and part of the electronic health record (EHR) at the LHCF, thus reducing repeat testing and enabling more timely and efficient clinical evaluation (See Figure 1).
Every effort should be made on a local or regional level to encourage this. This should include, as necessary, the changing of institutional policy/procedures and inter-institutional co-operation as well as petitioning authorities on changes/exceptions to any confounding regulations.
Intra- and inter-institutional testing: Within a single institution, laboratory testing for clinical diagnosis and treatment (both POCT and central laboratory) should, from a technical perspective, be overseen by the clinical laboratory and its leadership, but with overall guidance and needs evaluation from a cross-functional institutional committee. Budgetary issues are separate, but technically and from a legal operational perspective, the laboratory is where the expertise and responsibility reside.
Meeting both the clinical treatment and patient needs for testing, even with an effective and efficient central laboratory, are not always feasible in a single location — and so we have POCT for the wound care clinic, transplant clinic, oncology, HIV/AIDS, and the OB/GYN clinic. Each clinic/care center’s patients can be seen, assessed, and treated as quickly and efficiently without patient inconvenience or disruption in the central lab’s in-patient routine test processing. There is no single best way to do this; for some situations, the clinic may just need a dedicated phlebotomist and a dipstick lab with a pneumatic tube for specimen transport. For other situations, more sophisticated testing may be performed by professional laboratorians, nurses, or medical assistants. Whatever the choice for your institution’s situation(s), all is linked by the patient health record so a clinic visit is compatible with the rest of the institution and available for reference in case of later, e.g., hospital care.
References
1. CLSI- GP26-A4, Quality Management System: A Model for Laboratory Services. Clinical and Laboratory Standards Institute. Wayne, PA.
2. Elser R, Hess D, Moran RF. Assessment of the agreement between duplicate whole blood measurements of blood gases and pH on independently calibrated analyzers. Methodology and Clinical Applications of Electrochemical and Fiber Optic Sensors. 1990;11.
3. Moran, RF. Computer Standards, Healthcare Management Briefs. 1990.
4. Villanova PA. Standardization of Sodium and Potassium Ion-Selective Systems to the Flame Photometric [Reference Method: Approved Standard NCCLS Document C29-A2.; 2000.
5. Burnett R, Ehrmeyer SS, Moran RF, vanKessel A. Blood gas and pH analysis and related measurements; Approved Guideline. NCCLS C46-A. Published online 2001.
6. Moran RF. Point-of-care vs central Lab "discrepancies": Getting the message across. J Appl Lab Med. 2017;1(5):595-597. doi:10.1373/jalm.2016.021485.
7. Moran RF, Liesching TN. The ABC’s of ABG’s: A Cyclopedic Dictionary of the Testing Terms Used in Critical Care.; 2018.
8. Nichols JH, Alter D, Chen Y, et al. AACC guidance document on management of point-of-care testing. J Appl Lab Med. 2020;5(4):762-787. doi:10.1093/jalm/jfaa059.
9. Nichols JH. Utilizing point-of-care testing to optimize patient care. EJIFCC. 2021;32(2):140-144.
10. Moran RF. POC testing and reporting of sodium, and other small molecules need modified IFCC source/type designations to improve operational efficacy and for clinically accurate, unambiguous reporting from LIMS and HIS. EJIFCC. 2023;34(4):271-275.
11. Jenkins J. What can go wrong with point-of-care testing? CLN. Published July 1, 2023. Accessed June 25, 2025. https://myadlm.org/cln/articles/2023/julaug/what-can-go-wrong-with-point-of-care-testing.
12. Moran RF. Moran RF. POC testing and reporting of sodium, and other small molecules need modified IFCC source/type designations to improve operational efficacy and for clinically accurate, unambiguous reporting from LIMS and HIS. EJIFCC. 2023;34(4):271-275.
13. Moran RF. Overcoming data management challenges: The biggest challenge of all is….us. Medical Laboratory Observer. January 29, 2024. Accessed June 25, 2025. https://www.mlo-online.com/information-technology/data-management/article/53081209/overcoming-data-management-challenges-the-biggest-challenge-of-all-isus.
14. Moran RF. Point-of-care testing — Managing change when you are not in charge. Medical Laboratory Observer. July 22, 2024. Accessed June 25, 2025. https://www.mlo-online.com/continuing-education/article/55094063/point-of-care-testing-managing-change-when-you-are-not-in-charge.
15. Moran RF. Use of IFCC/IUPAC format for specimen and test method display in the electronic health record facilitates increased accuracy of information. J Appl Lab Med. 2025;10(3):738-742. doi:10.1093/jalm/jfae112.
16. Moran RF, Posada-Vergara MP. Critical care testing: The laboratorian in the emergency room. Medical Laboratory Observer. April 21, 2025. Accessed June 25, 2025.
About the Author

Maria Paulina Posada-Vergara, MD, MSc
received her medical degree from the National University of Colombia and her MSc from the University of Sao Paulo, SP, Brazil. She is an Infectious Diseases Fellow from Instituto de Infectologia Emilio Ribas, Sao Paulo, Brazil. Her more than 25 years of clinical practice have included health provider training and service implementations in urban and remote settings. Dr. Posada-Vergara’s extensive expertise in delivering healthcare in low-income settings across Africa and Latin America has focused on HIV/AIDS, hepatitis, tuberculosis, STIs, and tropical diseases.

Robert F. Moran, PhD, FCCM, FIUPAC
is the Principal Scientist at mviSciences, a consulting and educational services organization and President of AccuTest™ Proficiency Testing Services. Dr. Moran served multiple terms on the NCCLS (Now CLSI) Board of Directors and was an active participant or chairholder in several of their blood gas and electrolyte standards-writing teams. Also active in clinical chemistry internationally, he is an appointed Fellow of the International Union of Pure and Applied Chemistry (FIUPAC). He is a retired professor of chemistry and physics from Wentworth Institute of Technology but remains active in consulting work and writing.