Advances in POCT technologies outpace regulatory and accreditation requirements
Long after their deaths, two famous scientists continue to challenge us with their words. “Knowing is not enough; we must apply. Being willing is not enough; we must do,” said Leonardo da Vinci, Italian artist, scientist, and inventor. “The true sign of intelligence is not knowledge but imagination,” spoke German-born theoretical physicist Albert Einstein. Their pioneering work has influenced all areas of science and, perhaps, even science fiction.
The imaginary tricorder in the Star Trek series may represent the ultimate goal of integrated point-of-care diagnostics, but it remains a fictional object. However, the Internet of individual care (and with it, the creation of high volumes of clinical data), where sensors, tests, and wearable devices have moved out of the laboratory and clinic directly into our lives for self-management and remote monitoring, has already begun and presents significant challenges to providers, regulators, and accreditation agencies alike.
One great example of an actual device that demonstrates the power of decentralized healthcare, using an in vitro whole blood diagnostic test, is self-monitoring of blood glucose via blood glucose meters or wearable continuous glucose monitors to manage glycemic control. As evidenced by competitive funding of technologies to disrupt and transform traditional healthcare delivery, this paradigm shift will continue to advance POCT, self-testing, and remote monitoring of patients. The Qualcomm Tricorder X Prize, a $10-million award in the United States, seeks to spur the development of a “portable, wireless device in the palm of your hand that monitors and diagnoses your health conditions.” The Nokia X Challenge, a $2.25-million award also in the U.S., encourages breakthroughs in sensor technology to “bring about entirely new ways to monitor, access and improve consumer health.”
These challenges are interesting and exciting, and today we are already seeing a paradigm shift to patient-centric healthcare outside of the hospital driven by these new, emerging POCT technologies. However, to fully utilize these new technologies, which are rapidly transforming global healthcare, our current systems for approving and monitoring device performance will also require radical reform. Current regulatory and accreditation requirements were not originally developed for POCT but for laboratory-centered testing practices more than 30 years ago. They require further modification to meet the increasing demands for more testing in ambulatory settings, the urgent care clinic, and the home to improve therapeutic turnaround times for chronic disease management.
Technologies and regulations
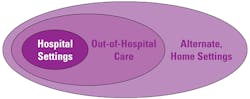
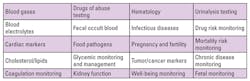
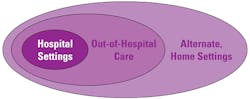
POCT technologies are evolving, and this market segment is growing at a rapid pace. Previously in the pages of MLO, I outlined how the role of POCT was beginning to impact the development of patient-centric care.1 The use of POCT has evolved further since 2013, with the range of professional and self-monitoring tests, including wearable devices, expanding significantly into the ambulatory setting with a patient-centric focus (Tables 1 and 2, and Figure 1). The need to improve patient safety and outcomes through rapid testing with trustworthy and actionable results at the point of care has provided significant benefits, including the ability to provide immediate and appropriate medical intervention.
Diagnostics has come a long way since the development of urine test strips and other lateral flow tests that advanced POCT, but the rules for approving and monitoring POCT performance and accreditation need to be re-evaluated. CLIA ‘88 was implemented almost 30 years ago, and the classification and requirements for assessing POCT devices has not kept pace with the development of these new technologies. CLIA ‘67 and ‘88 were developed to establish who could perform a diagnostic test with device classifications varying from CLIA-waived to moderate- and high-complexity testing. The intention of CLIA-waived status was to allow non-laboratory personnel such as physicians, nurses, patient care assistants, or the patient to perform a test (e.g., urine pregnancy, routine urinalysis, stool occult blood, or glucose).
Now patients, particularly those with an underlying chronic disease, have the opportunity to perform self-testing with a wide range of markers that can be monitored by either the patient or the clinician via a telemedicine hub. Today, the annual number of global blood glucose monitoring tests exceeds 5.6 billion, with the majority of testing being performed by the individual in the ambulatory setting. CLIA-waived tests have become more commonplace in the hospital setting as well, yet many of the requirements for accreditation and proficiency testing to assess post-market performance are based on systems developed for moderate- and high-complexity testing devices. Hospital requirements for CLIA-waived devices differ from self-testing and are based on testing requirements developed for laboratory test systems. CLIA-waived self-testing does not require quality assessment (QA) or proficiency testing (PT), but in the hospital both QA and PT are required.
In October 2016, the U.S. Food and Drug Administration (FDA) published new guidelines for manufacturers which, for the first time, separately defined the requirements for self-monitoring blood glucose (SMBG) devices and blood glucose monitoring systems (BGMS) for professional or prescription use in the hospital or other professional care settings.2,3 While the FDA, along with the Centers for Medicare and Medicaid Services (CMS), has taken the lead in addressing the differences in the use of self-monitoring and professional POCT devices by establishing new performance criteria, the accrediting and standards agencies have not yet kept pace with this initiative. The FDA held a public forum in March 2010 to addresses concerns about glucose meters in both the hospital and ambulatory settings. It took six years for the development and implementation of these new guidelines. Once the draft guidelines were published in January 2014, what followed was more discussion, and providers misunderstood that these guidelines were for manufacturers, not for hospital point-of-care programs.4,5 Other national and international bodies producing glucose performance guidelines, and standards still need to address the initiatives implemented by the FDA.6-8
QC and PT
Now that FDA performance guidelines have clearly differentiated both types of CLIA-waived devices (SMBG and BGMS), will QC and proficiency testing also be different? Are manufacturers’ recommendations sufficient in either the hospital or ambulatory settings, or will it be necessary to develop new approaches? Based on review of the 510(k)s for proficiency test (PT) materials, the products (device) were cleared in the mid-1980s and were labeled for assessing the imprecision of a device within a peer group and not for cross-comparison of multiple devices. Hospital proficiency testing programs and materials for monitoring whole blood POCT glucose are based on artificially manufactured materials which do not adequately evaluate or represent whole blood testing. Nor are these materials commutable to a higher standard such as isotope dilution mass spectrometry (IDMS) glucose. Many of the materials are bovine albumin-based matrices that do not adequately reflect human whole blood. Additionally, these PT (EQA) specimens do not take into account the complexity of the unique design and technology platforms that were designed to improve the accuracy of whole blood testing.9
While hospital blood glucose systems are CLIA-waived devices, either as self-monitoring devices or those that have earned 510(k) CLIA-waived status, and quality control and proficiency testing are required by CAP and JCAHO to obtain accreditation, these requirements do not apply to glucose SMBG in the ambulatory setting. Is this a contradiction in terms? Are hospital-based traditional quality and proficiency regimens really applicable now?10 How can we reconcile the uniqueness of hospital and ambulatory settings when developing new approaches to assessing quality? Is there evidence to support the need for changing the approach to assessing the quality and performance of POCT? Data exist to specifically answer these questions and to provide more insight about how to properly assess the performance of POCT, but an examination of the data goes beyond the scope of this article.
The way forward?
Is it time for manufacturers, physicians, laboratorians, regulators, and standards personnel to collaborate and design new schema for POCT whole blood device performance evaluation, monitoring, and certification? From the collective wisdom of these stakeholders, approaches can be devised that will establish better programs for assessing the quality and long-term proficiency of single-use POCT devices to ensure public and patient safety. POCT has now expanded to the use of wearable continuous glucose monitoring (CGM) devices. CGM is an integral component in the development of an artificial pancreas and, at the time of this publication, there are no criteria or proposed proficiency programs for evaluation of CGM performance. What are the performance requirements for these CGM devices? Are the traditional laboratory performance criteria applicable for assessing them? Should criteria be based on plasma equivalence (molarity) or activity (molality)?
In any event, it is important for us to go beyond the traditional and historical—as we have been challenged by da Vinci and Einstein—and develop new approaches to assess device performance from a clinical perspective. It is important to have this perspective as new technologies enter the market and affect patient care. Performance criteria for these devices must change to assess the quality of test results not just in real-time but continually over time. The time has come for us to develop new schema based on the significant volume of patient data generated by these devices at the point of care versus a post-facto proficiency testing method using a manufactured artificial substitute for whole blood.
REFERENCES
- DuBois J, The role of POCT and rapid testing. MLO. 2013;45(9):18-22.
- Department of Health and Human Services, Food and Drug Administration, Center for Devices and Radiological Health. Self-monitoring blood glucose test systems for over-the-counter use. October 11, 2014. http://www.fda.gov/downloads/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm380327.pdf.
- Department of Health and Human Services, Food and Drug Administration, Center for Devices and Radiological Health. Blood glucose monitoring test systems for prescription point-of-care use. October 11, 2014. http://www.fda.gov/downloads/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm380325.pdf.
- Department of Health and Human Services, Food and Drug Administration, Center for Devices and Radiological Health. Self-monitoring blood glucose test systems for over-the-counter use—draft guidance. Silver Spring, MD: FDA, January 7, 2014. http://www.fda.gov/downloads/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm380327.pdf.
- Department of Health and Human Services, Food and Drug Administration, Center for Devices and Radiological Health. Blood glucose monitoring test systems for prescription point-of-care use—draft guidance. Silver Spring, MD: FDA, January 7, 2014. http://www.fda.gov/downloads/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm380325.pdf.
- Clinical and Laboratory Standards Institute. POCT12-A3. Point-of-care blood glucose testing in acute and chronic care facilities; approved guideline—third edition. Wayne, PA: 2013.
- International Standards Organizations. ISO 15197:2013. In vitro diagnostic test systems—requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus (ISO/TC 212). Geneva, Switzerland: 2013.
- American Diabetes Association. Self-monitoring of blood glucose. Diabetes Care. 1996;19 Suppl 1:S62-6.
- Jacobs J, Fokkert M, Slingerland R, De Schrijver P, Van Hoovels L. A further cautionary tale for interpretation of external quality assurance results (EQA): Commutability of EQA materials for point-of-care glucose meters. Clin Chim Acta. 2016;462:146-147.
- CAP Memo Waived Glucose and INR PT Discontinuance, October 28, 2016, http://www.pointofcare.net/Arizona/102816_CAP_Memo_Gluc_INR_PT_Discontinuance.pdf.
Jeffrey A. DuBois, PhD, HCLD/CC (ABB) FACB, serves as Vice President, Medical and Scientific Affairs, for Nova Biomedical Corporation.
About the Author