Quality in, quality out: Practical considerations for ‘digital ready’ slides
Optimizing lab workflows to produce high-quality ‘digital ready’ slides for whole slide images (WSIs) is essential for organizations using digital pathology. A growing body of peer-reviewed evidence documents the benefits of digital pathology for clinical use, including enhanced accuracy and precision, the ability for digital images to be uploaded and reviewed remotely by multiple pathologists, and the acquisition and processing of large and complex datasets — ultimately resulting in a more efficient workflow, a better experience for pathologists and lab personnel, and improved patient care.
The last two decades have seen significant advances in digital pathology to the point where the acquisition speed, image quality, and infrastructure to support WSIs is at a level of maturity to facilitate routine, on-screen diagnostics.1-3 Moreover, there is growing acceptance for the use of digital pathology in the clinical setting in both normal times and public health emergencies such as the COVID-19 pandemic.4-6 Further, development of AI for pathology applications is growing rapidly and typically requires large datasets of WSIs for training and validation.7,8
High-quality WSIs are the foundation for clinical utilization of digital pathology and the building blocks for enablement of histopathology AI tools. Collation of WSI datasets is facilitated by the creation of so-called ‘digital ready’ slides, which are optimized for whole slide imaging. Capturing ‘digital ready’ slides involves more than installing a scanner in a lab. Standardization of histological slide preparation requires focus on both optimization of individual workflow steps as well as a holistic understanding of the complete process from sample acquisition to diagnosis. Knowing this in advance and taking appropriate steps to effectively support change management can promote engagement of stakeholders and pave a path to success.
We’ve learned from our work with organizations around the world that each step in the pre-scan process can be optimized for WSI — from embedding, microtomy and tissue placement to slide labeling, staining, and coverslipping. Doing so involves professionals across the lab establishing and adhering to agreed-upon standards. Technical University Munich began its journey to digital in 2013 and has identified several lab workflow steps imperative to efficient digitization of high quality WSIs. "For the workflow to remain efficient, we provide recommendations to the lab in terms of slide preparation,” says Viola Iwuajoku, Digital Pathology Team Lead at Technical University Munich. “For example, they need to be sure the coverslips are intact, that the barcodes are clearly readable, and that the slides are properly dried and no longer sticky from the embedding and staining processes.”
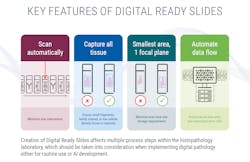
I concur these factors are essential, adding the importance of creating slides that embed all tissue in the smallest area possible on a slide — ideally in one focal plane — including tissue fragments, faintly stained tissue, or paucicellular tissue. Doing so can help labs refine and reduce scan time per image, limit scanner downtime due to jammed or broken slides, minimize image file size, and address file storage limitations, among other benefits (See Figure 1).
Image quality is of utmost importance for pathologists to make an accurate and confident diagnosis. Simple changes to how we prep slides can aid the adoption of digital pathology and highlight its many benefits including efficient review of cases, ease of collaboration with specialists, and remote work flexibility.
References
1. Fraggetta F, Garozzo S, Zannoni GF, Pantanowitz L, Rossi ED. Routine digital pathology workflow: The Catania experience. J Pathol Inform. 2017;8(1):51. doi:10.4103/jpi.jpi_58_17.
2. Williams BJ, Hanby A, Millican-Slater R, Nijhawan A, Verghese E, Treanor D. Digital pathology for the primary diagnosis of breast histopathological specimens: an innovative validation and concordance study on digital pathology validation and training. Histopathology. 2018;72(4):662-671. doi:10.1111/his.13403.
3. Hanna MG, Reuter VE, Ardon O, et al. Validation of a digital pathology system including remote review during the COVID-19 pandemic. Mod Pathol. 2020;33(11):2115-2127. doi:10.1038/s41379-020-0601-5.
4. Cap.org. Accessed September 26, 2023. https://documents.cap.org/documents/COVID19-Remote-Sign-Out-Guidance-vFNL.pdf.
5. Centers For MM. Clinical Laboratory Improvement Amendments (CLIA) Laboratory Guidance During COVID-19 Public Health Emergency.; 2020.
6. The Royal College of Pathologists. Digital pathology. Rcpath.org. Accessed September 26, 2023. https://www.rcpath.org/profession/digital-pathology.html.
7. Mccarthy J, Minsky ML, Rochester N, Shannon CE. A proposal for the Dartmouth summer research project on artificial intelligence. Artificial Intelligence: Critical Concepts. 1955;2:44-53.
8. Kanavati F, Toyokawa G, Momosaki S, et al. Weakly-supervised learning for lung carcinoma classification using deep learning. Sci Rep. 2020;10(1):9297. doi:10.1038/s41598-020-66333-x.
About the Author

Rob Monroe, MD, PhD
is a pathologist currently serving as Chief Medical Officer for Leica Biosystems and Chief Scientific Officer, Oncology, for Danaher Diagnostics. During his industry career, Rob also co-founded OncoMDx, a molecular diagnostics laboratory subsequently acquired by Core Diagnostics in 2012 where he served as Chief Medical Officer of the molecular lab and an international digital pathology service. Before joining industry, Rob practiced pathology in the Central Coast of California following a Medical Scientist Training Program Scholarship at Harvard Medical School and residency training in Anatomic Pathology at UCLA and Clinical Pathology at Stanford. Rob is board certified in Cytopathology, Anatomic Pathology, and Clinical Pathology and holds a PhD in Genetics.