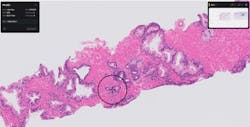
The U.S. Food and Drug Administration (FDA) approved the use of a software product to assist pathologists in the detection of prostate cancer.
The software, called Paige Prostate, is artificial intelligence (AI)-based software designed to identify an area of interest on the prostate biopsy image with the highest likelihood of harboring cancer, so it can be reviewed further by a pathologist. It is designed to be used as a supplement to pathologists’ review of digitally scanned slide images from prostate biopsies.
The FDA reviewed the device through the De Novo premarket review pathway, a regulatory pathway for low- to moderate-risk devices of a new type, and granted authorization of the software to Paige.AI, based in New York.
Paige.AI said the software is the first AI-based pathology product to receive De Novo approval from the FDA, allowing in vitro diagnostic (IVD) use via Paige’s FDA-cleared FullFocus digital pathology viewer.
The FDA approval “paves the way for the introduction of numerous future tools to help standardize pathology diagnosis, expedite the diagnostic process, and provide pathologists and patients greater comfort from the added scrutiny of their pathology slides,” said David Klimstra, MD, Co-Founder and Chief Medical Officer at Paige. AI.
According to the Centers for Disease Control and Prevention (CDC), aside from non-melanoma skin cancer, prostate cancer is the most common cancer among men in the United States. It is also one of the leading causes of cancer death among men.
The FDA evaluated data from a clinical study where 16 pathologists examined 527 slide images of prostate biopsies (171 cancer and 356 benign) that were digitized using a scanner. For each slide image, each pathologist completed two assessments, one without Paige Prostate’s assistance and one with Paige Prostate’s assistance. While the clinical study did not evaluate the impact on final patient diagnosis, which is typically based on multiple biopsies, the study found that Paige Prostate improved detection of cancer on individual slide images by 7.3% on average, compared to pathologists’ unassisted reads for whole slide images of individual biopsies, with no impact on the read of benign slide images.
Potential risks include false negative and false positive results, which is mitigated by the device’s use as an adjunct to the professional evaluation by a qualified pathologist who takes into account patient history, among other relevant clinical information, and who may perform additional laboratory studies on the samples prior to rendering a final diagnosis.