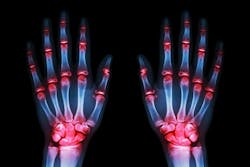
Reshaping the rheumatoid arthritis journey with smarter diagnostics
Autoimmune diseases occur when the immune system mistakenly attacks the body’s own tissues. They often present with symptoms like fatigue, joint pain, muscle weakness, and skin rashes, varying considerably between patients.1 They can also evolve over time, making them challenging for laboratory professionals and healthcare providers to accurately diagnose.
It takes an average of four doctors and four years for patients to receive an autoimmune diagnosis from the first visit.2 Delays in autoimmune disease diagnosis can be harmful — worsening disease progression, leading to unnecessary treatments, and reducing quality of life.3 In fact, 99% of individuals with autoimmune diseases report fatigue impacting their quality of life, while 89% say it also hinders their ability to work. 4 Studies show that delays in diagnosis not only worsen physical symptoms,3 but they also contribute to significant mental health challenges, such as anxiety, depression, and mistrust in the healthcare system. 5
Rheumatoid arthritis (RA), in particular, is one of the most common and debilitating autoimmune diseases, affecting about 1.3 million Americans.6 Timely and accurate diagnosis is critical, as early treatment can prevent irreversible joint damage and improve long-term outcomes. However, diagnosing RA remains complex since symptoms overlap with many other conditions and biomarkers are not always clear-cut, though advances in laboratory technology are beginning to close these gaps and help clinicians detect RA earlier and with greater accuracy.
Challenges associated with RA diagnostics
More than 15 million Americans live with one or more autoimmune diseases, 7 but up to 76% of autoimmune patients report at least one misdiagnosis.8 Diagnosing RA can be particularly challenging because the symptoms mimic many other conditions, and clinicians who are not specialists in identifying autoimmune diseases may not spot the signs early. RA symptoms, such as fatigue, joint stiffness, and pain, are often nonspecific and can resemble other inflammatory or autoimmune conditions. For RA specifically, one recent study found that 48.3% of patients with RA were misdiagnosed before presentation to a rheumatologist.9 Additionally, up to 20% of RA patients — likely even more, depending on the study — are seronegative, meaning that they are not going to test positive for the most relevant diagnostic markers.
With a shortage of rheumatologists combined with an increased need for these specialists, many patients visit multiple other healthcare providers before they reach a rheumatologist, prolonging their time to diagnosis. Primary care physicians are often the first point of contact for patients seeking care, but they may not have a high level of familiarity with autoimmune testing protocols.
Gender bias can also contribute to delays. In fact, women are 66% more likely to be misdiagnosed compared to men10 – a stark statistic, especially considering 80% of autoimmune conditions affect women.11 The above challenges all combine to contribute to longer diagnostic timelines that can delay or altogether halt patients’ access to effective treatment.
How combined testing assays are changing the diagnostic landscape
In 2010, the American College of Rheumatology (ACR) and the European League Against Rheumatism (EULAR) released new classification criteria for RA with two of the four classification domains involving blood tests. The key blood-based markers are rheumatoid factor (RF) and anti-cyclic citrullinated peptides (anti-CCPs). Testing for RF and anti-CCP antibodies is crucial in diagnosing RA. While RF is a common marker, anti-CCP is much more specific to RA. Based on these antibodies, RA in adults can be classified as either seropositive or seronegative.
In seropositive RA, blood tests show unusually high levels of antibodies called anti-CCPs. These are specific markers for RA and may show up as much as a decade before symptoms do, and around 60% to 80% of people diagnosed with RA have anti-CCPs.12 By definition, people with seronegative RA don’t have these antibodies in their blood. Most people who test positive for anti-CCP antibodies also test positive for RF. However, RF can also be elevated in many other conditions, such as chronic infections, liver disease, and other autoimmune disorders, making it a less specific marker for RA.
Because of its high specificity, anti-CCP testing is now considered the preferred diagnostic marker for RA. Clinicians often order both anti-CCP and RF tests together to improve diagnostic confidence and accuracy. Combining tests for RF and anti-CCP antibodies also significantly increases the accuracy of diagnosing RA. Measuring multiple antibody isotypes — such as IgA, IgM, and IgG — can also further enhance diagnostic sensitivity. The presence of several isotypes often indicates a more active immune response, which supports a serological diagnosis of RA. Researchers have found that testing for these RF isotypes and anti-CCP results in specificity of nearly 100%.
With a comprehensive RA profile, labs can cut through the uncertainty around RA, helping clinicians get to a diagnosis faster and have a more complete interpretation for the provider. As mentioned, time to diagnosis also shortens time to treatment, which can slow the progression of RA in patients. For example, disease-modifying anti-rheumatic drugs or steroids can help ease inflammation, and rehabilitative strategies like exercise are an important part of symptom management.
However, all of these interventions follow an accurate diagnosis, which means labs must be ready to provide the most comprehensive testing for better patient care. Systems that run this testing in parallel can improve diagnostic confidence without complicating a lab’s workflow. Providing the most comprehensive recommended testing means better support for clinicians trying to sift through RA symptoms, especially primary care physicians who may not be as familiar with autoimmune condition symptoms as rheumatologists are.
Progress toward faster, smarter, and more equitable RA diagnosis
The path to an accurate RA diagnosis has historically been long and uncertain, but new diagnostic criteria and technologies are changing this. These technologies not only increase laboratory efficiency, but also help physicians make faster, more confident decisions for patients.
Ultimately, closing the diagnostic gap in RA with these testing processes can help get patients to a faster diagnosis and intervention, improving their quality of life and giving them back time, mobility, and certainty in their care journey.
References
- Autoimmune diseases: types, symptoms & treatments. Cleveland Clinic. Accessed December 3, 2025. https://my.clevelandclinic.org/health/diseases/21624-autoimmune-diseases.
- Diagnosis tips. Autoimmune Association. Accessed December 3, 2025. https://autoimmune.org/resource-center/diagnosis-tips/.
- Kernder A, Richter JG, Fischer-Betz R, et al. Delayed diagnosis adversely affects outcome in systemic lupus erythematosus: Cross sectional analysis of the LuLa cohort. Lupus. 2021;30(3):431-438. doi:10.1177/0961203320983445.
- Ladd VT. Autoimmune fatigue: what does it feel like? Autoimmune Association. Accessed December 3, 2025. https://autoimmune.org/blog/beyond-tired-fatigue-and-autoimmune-disease/.
- Gunning JN. “But you don’t look sick:” Memorable messages of emerging adulthood autoimmune disease. J Soc Pers Relat. 2023;40(6):2008-2030. doi:10.1177/02654075221137548.
- Rheumatoid arthritis treatment. Brigham and Women’s Hospital. Accessed December 3, 2025. https://www.brighamandwomens.org/medical-resources/rheumatoid-arthritis-treatment.
- De Widt L. New study calculates autoimmune disease prevalence in U.S. Mayo Clinic. January 6, 2025. Accessed December 3, 2025. https://newsnetwork.mayoclinic.org/discussion/new-study-calculates-autoimmune-disease-prevalence-in-u-s/.
- Sloan M, Harwood R, Sutton S, et al. Medically explained symptoms: A mixed methods study of diagnostic, symptom and support experiences of patients with lupus and related systemic autoimmune diseases. Rheumatol Adv Pract. 2020;4(1):rkaa006. doi:10.1093/rap/rkaa006.
- Javaid U, Mahmud TH, Rasheed A, et al. Factors leading to diagnostic and therapeutic delay of rheumatoid arthritis and their impact on disease outcome. Cureus. 2023;15(1):e34481. doi:10.7759/cureus.34481.
- The Soliant Health 2024 State of Healthcare Report. Soliant Health. April 10, 2024. Accessed December 3, 2025. https://www.soliant.com/blog/the-soliant-health-2024-state-of-healthcare-report/.
- Goldman B. Stanford Medicine-led study shows why women are at greater risk of autoimmune disease. Stanford Medicine. February 1, 2024. Accessed December 3, 2025. https://med.stanford.edu/news/all-news/2024/02/women-autoimmune.html.
- Braschi E, Shojania K, Allan GM. Anti-CCP: A truly helpful rheumatoid arthritis test? Can Fam Physician. 2016;62(3):234.
About the Author

Tara Bruner, MHS, PA-C, DFAAPA
practices primary care at Primecare Medical Clinic in Searcy, AR and is Manager, Clinical Education, US for Thermo Fisher Scientific ImmunoDiagnostics Division. She received a Bachelor of Science in Biology degree from Oklahoma Christian University in 2000. After graduation she worked as a research technician in the department of Arthritis and Immunology at Oklahoma Medical Research Foundation. In 2005 she graduated from the University of Oklahoma Health Sciences Center with a Masters of Health Sciences in Physician Assistant Studies.