IHC biomarkers for breast cancer in the era of molecular testing
Molecular subtypes of breast cancer and differential gene expression patterns
Breast cancer is a heterogeneous disease and can be segmented in three subtypes based on IHC markers:
I. Hormone-receptor positive (i.e. Estrogen receptor (ER)/Progesterone receptor (PgR) positive)
II. Human Epidermal Receptor positive (i.e. HER2/neu-positive)
III. Triple-negative (ER/PgR-negative and HER2-negative)
These subtypes have significant implications in the management and treatment of breast cancer patients, particularly in the selection of trastuzumab.2 Recent advances in expression profiling technology have pushed this frontier further, resulting in additional molecular subtypes with distinct gene expression pattern as summarized in Table 1.
Why IHC?
It appears that the molecular determination of subtype may be able to provide more information than IHC in the clinical management of breast cancer patients. However, IHC is still widely used in the clinical setting. This is expected because:
- IHC is a well-established methodology, widely used in pathology labs around the world as compared to molecular (MDx) testing which in many parts of the world is still in its infancy. The College of American Pathologists (CAP) and the American Society of Clinical Oncology (ASCO) have jointly developed testing guidelines which are regularly updated with robust quality control (QC) measures in place to ensure an objective and accurate scoring of IHC results. An example is the latest update in HER2 testing for the requirement of concomitant IHC review for dual-probe ISH (in-situ hybridization) groups 2 to 4 to arrive at an accurate HER2 status designation.3
- While standard morphology examination of hematoxylin and eosin (H&E) slides by pathologists remains the preferred method in the diagnosis of breast cancer, IHC has become a validated and integral part of the testing algorithm. Heterogeneity of cancer makes spatial visualization by IHC important. IHC stains also enable pathologists to correlate the results with the morphological examination.
- IHC is also the best tool for clinical labs given the ease of analysis, low cost, and application to routine formalin-fixed paraffin-embedded (FFPE) samples combined with quick turnaround time. Advances in IHC automation, together with digital pathology capabilities, could empower high-throughput laboratories even further without the need to develop and standardize a totally new molecular workflow.
Utility of IHC biomarkers in diagnosing breast cancer
I. Identification of subtypes of breast cancer
It is apparent from Table 1 that the identification of breast cancer subtype is very important in the selection of the appropriate treatment and determining the prognosis of the disease. ER, PgR, and HER2 are routinely tested for this purpose and well established in ASCO/CAP guidelines. The cell proliferation marker Ki-67 may also assist in differentiating the luminal subtypes (luminal A vs. B) and the choice of hormone therapy with or without chemotherapy.
II. C-erb-B2 overexpression (aka HER2/neu)
Perhaps the best-known case for IHC testing, C-erb-B2/HER2/neu overexpression is indicative of sensitivity to anti-HER2 therapies, such as trastuzumab and lapatinib. Currently, IHC and FISH are the two clinical methods approved to evaluate overexpression at cell surface and HER2 amplification, respectively. Overexpression is typically the consequence of gene amplification and the two tests should correlate well.
III. Myoepithelial markers to differentiate invasive carcinoma from carcinoma in situ
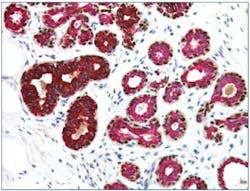
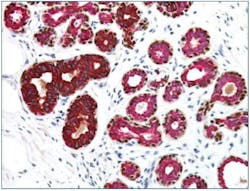
It can be challenging to distinguish ductal carcinoma in situ (DCIS) from invasive breast cancer by H&E stains alone. The utility of myoepithelial markers can be attributed to the fact that these markers should still be contained within ducts in DCIS, whereas the myoepithelial cell layer and basement membrane would have been breached in invasive carcinoma, no longer intact, and not stained in IHC. Typical myoepithelial markers include smooth muscle actin (SMA), calponin, p63, and smooth muscle myosin heavy chain (SMMHC).4 High-molecular-weight cytokeratins (CKs), such as CK14 and CK5/6, are relatively specific to myoepithelial cells; although, some of the CKs are also expressed in some invasive breast cancer such as basal-like.5 Jain et al. (2011) reported reduced interobserver and intraobserver variability in the diagnosis of atypical ductal hyperplasia using a cocktail of CK5, CK14, p63, CK7, and CK18 and demonstrated how IHC biomarkers multiplexing may benefit management of these cases.6 (Figure 1)7 The list of myoepithelial markers keeps growing, but there could be significant variation in sensitivity and specificity. One must exercise caution when interpreting the loss of myoepithelial markers, supported by abnormal morphologies in H&E slides.8
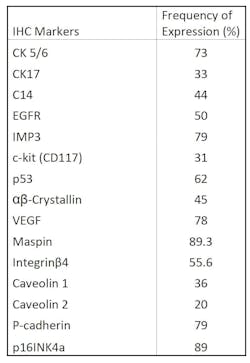
IV. IHC biomarkers in Basal-like Breast Cancers (BLBCs) and Triple-negative Breast Cancers (TNBCs)
V. Other IHC markers for prognosis/treatment selection10
Apart from the routine ER, PgR, and HER2 tests, the following IHC biomarkers have been implicated as predictive and prognostic tools in the management of breast cancers:
- Elevated Ki-67 may be a predictive factor for a higher rate of complete pathological response in neoadjuvant chemotherapy.11,12
- BCL2 and p53 expression may help to define high-risk patients in the use of adjuvant chemotherapy in early breast carcinoma.
Conclusion
Over the last decade, MDx tests such as multigene signatures and Next Generation Sequencing (NGS) have been seen as the rising star of cancer diagnostic testing. However, pathologists are not readily embracing this new technology for breast cancer. In the 2013 St. Gallen
Consensus Conference,13 most panelists (70 percent) were against multi-gene expression profiling for breast cancer subtype definition. In fact, the majority considered ER, PgR, and Ki-67 sufficient to differentiate between luminal A and luminal B HER2 negative cases. Sixty percent of the panelists did not believe subtype determination could be accomplished by MDx alone, and 64 percent believed histological grading was not a substitute for Ki-67.
This outcome confirms our earlier discussion that IHC is still an indispensable diagnostic tool for breast cancer. That said, it is essential to standardize the IHC protocol and ensure that variables such as fixatives, antibody manufacturer, and the type of immunostaining methods are under control. The choice of a reliable and established reagent provider with good quality control cannot be emphasized enough.
Although new MDx tests for breast cancer will keep entering the market and generate much excitement in the years to come, the mature and well-established IHC methodology continues to be the tried and true workhorse for the majority of pathologists for routine breast cancer diagnosis in the foreseeable future.
REFERENCES
- SEER Cancer Stat Facts: Female Breast Cancer. National Cancer Institute. Bethesda, MD. https://seer.cancer.gov/statfacts/html/breast.html. Accessed July 29, 2019.
- National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology (NCCN Guidelines): Breast Cancer Version 1.209. NCCN. https://www.nccn.org/professionals/physician_gls/pdf/breast_blocks.pdf. Published March 14,2019. Accessed July 31, 2019.
- Wolff A, Hammond M, Allison K, et al. American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. Arch Pathol Lab Med. 2018;142: 1364–1382.
- Zaha DC. Significance of immunohistochemistry in breast cancer. World J Clin Oncol 2014; 5(3): 382-392.
- Zhao L, Yang X, Khan A and Kandil D. Diagnostic Role of Immunohistochemistry in the Evaluation of Breast Pathology Specimens. Arch Pathol Lab Med. 2014;138:16–24.
- Jain R, Mehta R, Dimitrov R, Larsson L, et al. Atypical ductal hyperplasia: interobserver and intraobserver variability. Modern Pathology. 2011;24:917–923.
- Yeh IT, Mies C. Application of immunohistochemistry to breast lesions. Arch Pathol Lab Med. 2008;132(3):349-58.
- Zhao L, Yang X, Khan A and Kandil D. Diagnostic Role of Immunohistochemistry in the 14Evaluation of Breast Pathology Specimens. Arch Pathol Lab Med. 2014;138: 16–24.
- Leidy J, Khan A, Kandil D. Basal-Like Breast Cancer: Update on Clinicopathologic, Immunohistochemical, and Molecular Features. Arch Pathol Lab Med. 2014;138: 37–43.
- Zaha DC. Significance of immunohistochemistry in breast cancer. World J Clin Oncol 2014; 5(3): 382-392.
- Caudle AS, Gonzalez-Angulo AM, Hunt KK, Liu P, et al. Predictors of tumor progression during neoadjuvant chemotherapy in breast cancer. J Clin Oncol 2010;28: 1821-1828.
- Peter AF, Heusinger K, Haeberle L, Niklos M, et al. Ki67, chemotherapy response, and prognosis in breast cancer patients receiving neoadjuvant treatment. BMC Cancer 2011; 11: 486.
- Harbeck N, Thomssen C, Gnant M. St. Gallen 2013: brief preliminary summary of the consensus discussion. Breast Care (Basel). 2013;8(2):102–109.
- Kittaneh M, Montero AJ, Glück S. Molecular profiling for breast cancer: a comprehensive review. Biomark Cancer. 2013(5):61–70. Published 2013 Oct 29.
- Leidy J, Khan A, Kandil D. Basal-Like Breast Cancer: Update on Clinicopathologic, Immunohistochemical, and Molecular Features. Arch Pathol Lab Med. 2014;138: 37–43.
About the Author

Maria Marsh BS, MBA
serves as a Product Manager at Biocare Medical. She holds a BS in Biotechnology from the University of California, Davis and an MBA from
Saint Mary’s College of California.

Kwok-Fai Ng, BS
serves as Marketing Content Manager at Biocare Medical. He received his degree in biomedical science from the University of Technology, Syndey in
Australia.