Blood gases and pH measurement
As described in last month’s first part of this two-part article,1 lung dysfunction may be categorized as an airway-based, tissue-based, or blood circulation–based disease. These are intimately linked to renal functioning, both leading to the measurements we refer to as ‘blood gases.’ When oxygen (O2) reaches tissues, it enables the production of energy from carbon compounds and in doing so produces carbon dioxide (CO2) and water. As a gas, the CO2, diffuses from the tissue cells into the blood and is transported to the lungs where it dilutes the O2 in the incoming air and thus inhibits O2 transfer to the tissues. The primary measured gases, O2 and CO2, are essentially linked — each gas pressure is the driving ‘force’ in its environment. For a more comprehensive overview, I suggest the monograph by John Toffaletti, Blood Gases and Electrolytes.2
Oxygenation assessment in blood gas analysis
Complete laboratory evaluation of oxygenation often requires much more than simple blood gas measurements — most notably CO-oximetry plus analysis of specimens collected under specified conditions. Nevertheless, many patients can be evaluated and treated successfully using blood gases alone if clinical observations and patient history are considered. Clinically, measurement of oxygen partial pressure (pO2) is performed on whole blood, usually an arterial specimen. However, since other anatomic sources are clinically useful, a complete symbol should include that additional information. (See MLO August 2024 continuing education article, “Point-of-care testing: Managing change when you are not in charge.”)
Measurement of pO2 is based on the Clarke polarographic electrode and modified by Severinghaus and Bradley to include a semi-permeable membrane such that only the diffusible oxygen is measured in the electrode’s buffer based on the current flow produced at a set millivoltage characteristic for only oxygen.
O2 + 4e- + 4H+ à 2H2O
The oxygen partial pressure (tension) of arterial blood, pO2(aB) is the indicator of O2 diffusion into the pulmonary circulation — the driving force in moving O2 from one compartment to another (e.g., alveoli of the lungs to the pulmonary capillaries). Typical levels for pO2(aB) in a young, healthy adult is >95 mm Hg (12.7 kPa). Nevertheless, clinical action for hypoxemia is usually not taken unless the pO2 is <80 mmHg (10.7 kPa). Oxygen levels are decreased in cases of impaired lung function (chronic obstructive pulmonary disease [COPD]) or when there is low-inspired oxygen pressure (e.g., at high altitudes or in the presence of other gases). With age, the pO2(aB) lowers by about 1 mm Hg(0.13kPa) for each year beyond 60. Measures to improve pO2(aB) include optimizing mechanical ventilation (reduces pCO2) and increasing inspired O2 percentage - F1O2. Along with the total hemoglobin, the pO2 of arterial blood is the primary sign of oxygenation status for most patients.
Evaluating the blood gases alone in a healthy young adult living near sea level, the reference value for pO2(aB) is about 95 mmHg (12.7 kPa). As with pCO2 and pH, however, a wider range of values may occur before any therapeutic action is shown. A pO2 of 80mmHg (10.7kPa) signals therapeutically significant hypoxemia (low blood oxygen). Above 80mmHg, there is slight change in oxygen saturation or oxygen content with changes in oxygen partial pressure, but below 80 mm Hg, decreases in saturation occur rapidly. Normal deterioration of lung function with increasing age (over 60) causes a decrease in expected pO2 values of approximately 1.0 mmHg (0.13 kPa) per year.
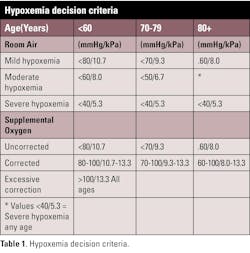
Oxygen status is commonly defined based on a patient's pO2 while breathing room air (21% O2). Estimates of hypoxemia can be made, even if the patient's ventilation and oxygen are being supported. An estimate of the expected pO2(aB) used by many clinicians for patients receiving oxygen therapy is based on multiplying the fraction of inspired oxygen (FIO2) by five (mmHg). If measurements are in kPa (SI), the FIO2 should be multiplied by two thirds (0.67). If a patient's measured value is lower than the estimated, hypoxemia on room air may be assumed, or a collection/measurement error may be suspected (See Table 1).
Clinically, if cardiac output and peripheral perfusion are considered adequate, then one or more of the following may be associated with a decreased tissue oxygenation: a.) oxygen saturation (gas exchange/shunting), b.) oxyhemoglobin fraction and total oxygen content of the blood (gas exchange and functional hemoglobin vs dyshemoglobins) or c.) P50 changes (indicates a tendency to accept and release oxygen from the blood hemoglobin.)
Oxygen saturation
Oxygen ‘saturation’ (sO2) is a common means of assessing oxygenation status, especially with the widespread use of peripheral or ‘pulse’ oximetry. While such devices give a true ‘saturation’ value, the use of their data to determine oxygen content or adequacy of oxygenation is inadvisable unless there is a clear understanding of the patient’s total hemoglobin (cHbt or ctHb) and dyshemoglobins such as carboxy and methemoglobin (COHb, MetHb), neither of which carries oxygen.
There are significant caveats in the clinical application of sO2 values. Consider the following:
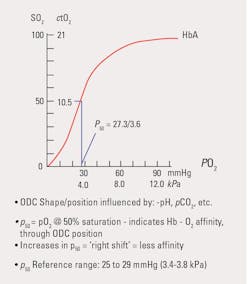
- The pO2 – sO2 relationship or ‘curve’ itself. The oxyhemoglobin dissociation ‘curve’ (ODC) (see Figure 1) is derived from the oxygen content-pO2 diagram in which there was no adjustment for the factors which affect the ‘curve.’ The result is the neatly smoothed and familiar sigmoid or s-shape seen in the ODC figure. Note the steep drop in the value for pO2 below sO2 of 90%. A small percentage change of sO2 can correspond to a substantial change in pO2, differences that are highly significant, but might not be recognized as such if monitoring the sO2 alone. Further, the p50 (partial pressure at 50% sO2), shows the tendency of hemoglobin to bind/release oxygen.
- Devices measuring only oxygen saturation (and pulse rate) cannot measure the presence of depleted oxygenation resulting from dyshemoglobins. In fact, consider carbon monoxide poisoning, treated at the scene of exposure with 100% oxygen, could result in a pO2 of >>100mmHg and a saturation of >>95%, while at the same time the patient is clinically severely hypoxic.
- Using the estimated saturation as found on standalone blood gas systems may be valid for healthy people. This approach was the consequence of early (1960s–1970s) attempts to explain partial pressure of oxygen versus the true saturation as was explained in textbooks of the time; unfortunately, some still report that ‘saturation.’ It’s a nice curve-fitting computation, but the variables and assumptions involved limit its utility except as a teaching tool, rather than clinical usefulness.
True oxyhemoglobin saturation
The saturation measurement performed before dedicated photometric technology (CO-oximetry) extracted oxygen gas manometrically from a patient blood specimen (oxygen content) followed by the same measurement on the same specimen exposed to pure oxygen.
CO-oximetry, the multi-wavelength photometers dedicated to measuring many hemoglobin derivatives simultaneously give results equivalent to the old and slow technique, but in seconds rather than in hours.
The key to understanding the estimated ‘saturation’ from standalone blood gas analyzers is that it was made available to explain pO2 values relative to the true saturation values, but not as a substitute for a measured saturation clinically. While some manufacturers of blood gas analyzers still include the estimated value, it should not be reported since it is likely to add to confusion when comparing with measured values.
Summary: Gas exchange, oxygen quantities
Blood gases (pCO2 and pO2) and pH and the relationships and definitions described relate to the laboratorian's ability to assess the quality of results obtained before they are recorded/reported, so the values obtained can be better evaluated. If they do not make sense, reanalysis or other consultation may be proper. By understanding these concepts and reviewing the data prior to reporting the results, an overall improvement in the quality of these most critical values can be obtained and delays minimized.Evaluation of a patient’s oxygenation status is best done while the patient is breathing room air, however, estimates of hypoxemia can be made if the patient's ventilation is being supported. If cardiac output and peripheral perfusion are considered adequate, then one or more of the following participate in the decreased tissue oxygenation: oxygen saturation, oxyhemoglobin fraction, total oxygen content, or P50. Measurements of COHb, MetHb, sulfhemoglobin, and 2,3-DPG can help find which parameter is tied to the decreased oxygenation. These additional measurements are essential for the rapid and complete evaluation necessary in the emergency/trauma environment.
References
- Moran RF. Blood gases and pH: Measurement and clinical overview. Medical Laboratory Observer. August 25, 2025. Accessed September 3, 2025. https://www.mlo-online.com/diagnostics/hematology/article/55306204/blood-gases-and-ph-measurement-and-clinical-overview.
- Toffaletti, John D, Blood Gases and Electrolytes, AACC Press, 2009 (2nd Ed), ISBN 13: 978-1-59425-097-2 and ISBN-10: 1-59425-097-9.
- Moran RF, Liesching TN, The ABC’s of ABG’s: A Cyclopedic Dictionary of the Testing Terms Used in Critical Care. Momentum Press®, LLC, ISBN-13: 978-1-94708-348-6 (print), ISBN-13: 978-1-94708-349-3 (e-book).
About the Author

Robert F. Moran, PhD, FCCM, FIUPAC
is the Principal Scientist at mviSciences, a consulting and educational services organization and President of AccuTest™ Proficiency Testing Services. Dr. Moran served multiple terms on the NCCLS (Now CLSI) Board of Directors and was an active participant or chairholder in several of their blood gas and electrolyte standards-writing teams. Also active in clinical chemistry internationally, he is an appointed Fellow of the International Union of Pure and Applied Chemistry (FIUPAC). He is a retired professor of chemistry and physics from Wentworth Institute of Technology but remains active in consulting work and writing.