Blood gases and pH: Measurement and clinical overview
Acid-base homeostasis, while resulting from complex interactions of biochemistry and physiology, boils down to lung and renal function/dysfunction responding to the rest of the body’s production of acids and bases in health and disease. Since the lungs are the first responders of the mammalian body, this is typically categorized as airway-based, tissue-based, or blood circulation–based. These are intimately linked to renal function, both leading to the measurements we refer to as ‘blood gases’ (pH, pO2 and pCO2) and related measurands.
Collecting the specimen
The predominant specimen used for measuring pH, pO2 and pCO2 is from an arterial or arterialized capillary bed collection. Because of different susceptibility to collection errors, the specimen should be differentiated on the report using for example pH(aB) or pO2(caB) and should be collected using dry, soluble Li Heparin (not therapeutic heparin) in the collection device. Measurements should be made within 10 minutes of collection rather than being placed on ice and batch tested.
Measuring the blood gases and pH
For pH alone and pCO2, the principle of measurement has changed little in the more than 50 years since John Severinghaus and Greeman Bradley applied a silastic membrane to a pH electrode (and a cellophane membrane to a Clarck electrode) in the mid-1950s. The glass of a pH electrode integrates lithium into its silicate matrix separating a buffer and electronics from the blood specimen. As the hydrogen ion levels vary, the voltage across the glass changes, reflecting changes in pH. The silastic membrane on the CO2 electrode separates the blood from another buffer on the outside of a pH electrode so the change in pH of that buffer represents only the changing pCO2. For oxygen, the cellophane membrane on the oxygen electrode separates the blood from a buffer in which a set voltage is applied forcing the stoichiometric conversion of oxygen to current. With calibration, these voltages/currents are converted to the appropriate values. The same concepts are used to measure other ions and molecules, (e.g., a pO2 electrode is at the heart of many glucose meters, both whole blood and continuous.
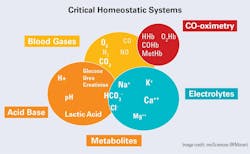
Critical homeostatic systems
Figure 1 shows the essentials of the human homeostatic systems. Notably, the blood gases are of themselves only a small part. However, the primary measured gases are essentially linked to all, especially the acid-base (pH). Each gas is the driving force in the system.
The primitive base-to-acid ratio (~20:1) has not only survived inside many species but has become critical for the proper functioning of the whole organism. This is reflected in the role that hydrogen ion concentration plays in many metabolic systems including the extraction of energy from food, the maintenance of cell and tissue integrity and oxygen transport and delivery.
While in various texts, each system is typically discussed on an individual basis for the sake of conceptual understanding, they are all fundamentally related. For a comprehensive but brief overview, this author suggests the monograph by John Toffaletti, “Blood Gases and Electrolytes.”
Acid-base: Hydrogen ion (pH), bicarbonate, and carbonic acid
The human body continually produces acid as it produces energy. Much of this acid is in the form of carbon dioxide (CO2), the end-product of aerobic metabolism which is akin to combustion — the burning metabolically of carbohydrates and related compounds resulting in CO2 and water. The CO2 is eliminated by the lungs at an approximate rate of almost 300 liters per day per human.
In addition to CO2, a typical ‘western’ mixed diet produces metabolic acids such as phosphoric and sulfuric acids- referred to as "fixed" acids since they cannot be converted to CO2. Dietary bases produced metabolically neutralize some of the acids, but the rest must be neutralized by the body so that acid-base homeostasis is maintained.
Acids produced as a part of aerobic metabolism are, in general, either carbonic acid (resulting from the direct metabolism of carbohydrates) or ketoacids (acetoacetate, hydroxybutyrate). The carbonic acid results from dissociation of the dissolved CO2 into its components. Ketoacids, however, dissociate directly into hydrogen ions (H+) and the corresponding anions. The result is the dynamic equilibrium between the various anions and the hydrogen ions. The sources of specific H+ are indistinguishable, and the H+ status can usually be assessed by an understanding of the carbonic acid/bicarbonate system alone.
Increasing hydrogen ions = Metabolic changes
Hydrogen ions produced by metabolic action will cause a decrease in blood pH if "buffering" systems are not in place and functioning. Because changes in pH in either direction can alter the H+ impact on enzyme-catalyzed equilibria, various enzyme systems can be significantly affected. Additionally, the dynamic equilibria set up between hemoglobin and oxygen is also influenced by pH. Further, in situations involving tissue hypoxia, more acid is produced (e.g., lactic acid), putting the organism at a further disadvantage (e.g., multiple organ failure).
With these several examples in mind, all of which can be occurring in any one patient, it is simple to see just how critical the understanding and assessment of acid-base status is to the physician and the laboratorian both.
Acid-base homeostasis
Buffers are used by the human body to support the hydrogen ion levels/pH within a necessary range for effective functioning of the many metabolic processes. The pulmonary and renal organ systems are two organs that ‘buffer’ changes in pH. However, neither are chemical buffers. Chemical buffers are substances that minimize changes in the pH of a solution when strong acids or bases are added. A major biologically important chemical buffer is the carbonic acid/bicarbonate pair, not only because it is quantitatively the largest at more than 50% of the total, but also because the bicarbonate system is integrally related to the pulmonary and renal systems, and it can be readily measured in the blood.
Chemical buffers present in the tissues/fluids react at once to neutralize any acid produced. However, two secondary mechanisms for "buffering" are significant — the fast-acting pulmonary system, (minutes to hours), and the renal system (hours to days). Their actions are summarized in Figure 2.
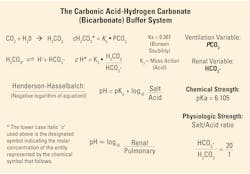
Significance of the carbonic acid–bicarbonate buffer system
When the carbon dioxide produced in tissues during metabolism dissolves in the aqueous intra/extra cellular fluids and by diffusion dissolves in the blood, it forms an equilibrium according to the basic chemical Law of Mass Action as shown in Figure 2. The amount of carbonic acid formed equals the product of the solubility and unit conversion constant KS, and the partial pressure of the carbon dioxide gas produced.
As may be seen in Figure 2, the Henderson-Hasselbalch relationship enables the evaluation of the acid-base data (pH) from a blood gas report with respect to both primary acid-base disorder and compensating responses.
Clinical implications of blood gas measurands
The amount of hydrogen ion (measured pH) in the blood is key to the interpretation of a blood gas report since it answers the basic question of if the condition is acidosis or alkalosis. Changes in the measured pCO2 leading to changes in pH come next. Increases in pCO2 result from hypoventilation with a later increase in carbonic acid and hydrogen ions (decrease in pH). Thus, a decrease in pH due to an increase in pCO2 (hypoventilation), is classified as a pulmonary or respiratory acidosis. The reverse, a decrease in pCO2 due to hyperventilation, results in a pulmonary or respiratory alkalosis because of the decreased carbonic acid and later increase in pH.
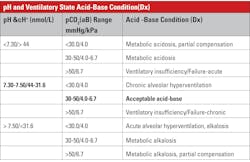
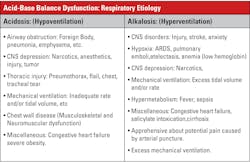
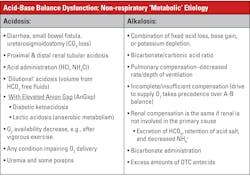
Acidosis-alkalosis categories and measured values
Table 1 summarizes acid-base status based on the measured values from a blood gas system — the quantities known with greatest certainty — pH and pCO2, rather than values for other quantities such as base excess, which arise from assumptions and calculations. A caveat regarding Table 1: Medically, ventilatory failure implies decreased ventilation, which causes increased CO2 and H ions and decreased pH. If the respiratory system is responding to metabolic acidosis by trying to blow off CO2, it will never blow off enough to normalize pH above 7.4 assuming a simple blood gas disorder. If there were a second coexisting abnormality (double gas disorder) then the pH could increase above 7.4.
Some of the clinical implications of the acid-base disorders are shown in Tables 2 and 3. The important thing to recall is the broad-based application of acid-base status; as to a great extent, that status reflects many serious conditions but is not typically a diagnosis in itself.
See the October issue for part two of this two-part series for a discussion of the final measurand-oxygen.
References
- Toffaletti JG. Blood Gases and Electrolytes. 2nd ed. American Association for Clinical Chemistry; 2009.
- Moran RF, Liesching TN. The ABC’s of Abg’s(Tm): A Cyclopedic Dictionary of the Testing Terms Used in Critical Care. Momentum Press; 2018.
About the Author

Robert F. Moran, PhD, FCCM, FIUPAC
is the Principal Scientist at mviSciences, a consulting and educational services organization and President of AccuTest™ Proficiency Testing Services. Dr. Moran served multiple terms on the NCCLS (Now CLSI) Board of Directors and was an active participant or chairholder in several of their blood gas and electrolyte standards-writing teams. Also active in clinical chemistry internationally, he is an appointed Fellow of the International Union of Pure and Applied Chemistry (FIUPAC). He is a retired professor of chemistry and physics from Wentworth Institute of Technology but remains active in consulting work and writing.