Fanconi anemia: A diagnostic approach to diagnosis and treatment
Fanconi anemia (FA), also known as inherited aplastic anemia, is a disease that causes bone marrow failure and many physical abnormalities. FA is a rare, fatal disease that affects 1 out of 136,000 newborns and usually results in death before age ten if undiagnosed.1 FA is caused by a genetic mutation in a DNA repair pathway. As a result of this mutation, cell death increases in the bone marrow, which causes a decrease in red blood cells, white blood cells, and platelets. These cells help transport oxygen, fight infections, and clot blood. Therefore, without these cells, serious complications occur.
According to the National Heart, Lung, and Blood Institute, anemia is any condition leading to a shortage of red blood cells.2 There are different types of anemia, some not life threatening and some that are very serious. Fanconi anemia is a type of serious anemia that is chronic and has a strong genetic component. It is like DBA, Diamond-Blakfan anemia, and usually present with similar symptoms. One of the symptoms of DBA and FA is a failure of the bone marrow to produce an adequate supply of red blood cells due to bone marrow failure. This shortage causes the anemic state in patients and leads to an overall depletion of oxygen throughout the body.3 In addition, FA patients can experience some physical abnormalities that are sometimes associated with this disorder. Fanconi anemia is caused by FA complementation gene (FANC) mutations that significantly reduce genomic stability.4 There are 23 FANC genes found to be involved in DNA repair, and most are inherited recessively. Only two are X-linked or autosomal dominant: FANCB and FANCR.1
Diagnostic criteria
As previously stated, FA is only suspected after the development of pancytopenia, so the disease is far along when detected. Therefore, it is recommended to screen all children, or all young adults diagnosed with aplastic or hypoplastic anemia for FA.5 However, if parents undergo genetic testing and both are carriers of the FA gene, testing can be performed on the fetus. According to Soulier, “increases of fetal hemoglobin, serum alpha-fetoprotein, and macrocytosis are commonly noted in FA.”5 An increase in fetal hemoglobin is evident of excessive destruction of red blood cells, while alpha-fetoprotein helps indicate any chromosomal abnormalities, which are both characteristics of FA. Fetal hemoglobin and serum alpha-fetoprotein tests can be performed while the individual is pregnant or after birth. The primary genetic test for FA is a chromosomal breakage test. A chromosomal breakage blood test elicits the sensitivity of FA cells to DNA cross-links by exposing them to chemical cross-linking agents such as diepoxybutane. Since FA cells cannot break down these cross-links, the chromosomes will break, resulting in a positive chromosomal breakage test.5 Along with a positive chromosomal breakage test, a complete blood count (CBC) and bone marrow aspiration or biopsy can be performed to get a more precise diagnosis. If an individual has FA, pancytopenia will be seen on the CBC, and hypocellular bone marrow will be found on a bone marrow biopsy.1
Laboratory diagnosis
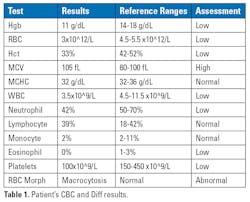
When assessing patients with FA, the hematology test and its values can be very helpful. Initial screening test results can help alert the pathologist that a bone marrow test may be needed to even assess the patient further. One example could be a low cell count on any of the following: white blood cell, red blood cell, platelets, etc. If leukopenia or thrombocytopenia is seen, then the pathologist may check for bone marrow failure or some other disease. The red blood cell morphology is often macrocytosis in several Fanconi patients with an average mean corpuscular volume (MCV) value of 105 fL. The CBC differential is usually normal.
Prognosis and treatment
The prognosis for individuals with FA is very poor. FA patients are highly susceptible to cancer and often develop acute myeloid leukemia (AML).4 The cause of death in patients with FA is usually bone marrow failure or malignancy.
Due to increased cell death, blood transfusions are a great supportive therapy for FA patients.1 A more long-term treatment, if successful, is a stem cell transplant. However, this procedure is invasive, so it is reserved as a last option. A new technique that could be beneficial is the correction of CD34+ in affected cells by gene therapy.1 CD34+ is a cell marker of hematopoietic stem cells, so correcting these mutated markers would fix the pancytopenia in FA patients.
Case study presentation
A 12-year-old boy presented with pallor and fatigue for the past month at the hospital. Upon inspection, he had moderate pallor, multiple light brown birthmarks, a palpable liver, and short stature for his age group. He had hypoplasia of the left thumb and a total absence of his right thumb.6 His family history was normal, as his parents and siblings were healthy. A CBC and differential were performed (Table 1), and hypocellular marrow was shown on a bone marrow biopsy. Fanconi anemia was suspected, so a chromosomal breakage test was performed, and it was positive. The positive chromosomal breakage test resulted in a FA diagnosis.6Conclusion
Fanconi anemia is a rare but deadly anemia that is caused by a genetic mutation. The mutation of the FANCA gene interferes with an important DNA repair pathway concerning interstrand cross-links. The toxic buildup of these links results in poor growth, hypocellular bone marrow, and pancytopenia. Diagnosis is often late in the development of FA, limiting the treatment available for these individuals. Further study into FA, specifically into the early stages of disease development, would aid in early diagnosis and help improve patients' quality of life.
References
1. Bhandari J, Thada PK, Puckett Y. Fanconi Anemia. StatPearls Publishing; 2022.
2. What is anemia? NHLBI, NIH. Accessed August 22, 2023. https://www.nhlbi.nih.gov/health/anemia.
3. http://www.ghr.nlm.nih.gov/condition/diamond-blackfan-anemia
4. Peffault De Latour R, Risitano AM, Gluckman E. Cogenital and Acquired Bone Marrow Failure. (Aljurf MD, Gluckman E, Dufour C, eds.). Elsevier; 2017.
5. Soulier J. Fanconi anemia. Hematology Am Soc Hematol Educ Program. 2011;2011:492-7. doi:10.1182/asheducation-2011.1.492.
6. Roy M, Bala AK, Roy D, Pandit N. Growth hormone deficiency in fanconis anemia. Indian J Hematol Blood Transfus. 2013;29(1):35-8. doi:10.1007/s12288-011-0119-6.
About the Author

Floyd Josephat, Ed.D., MLS(ASCP)
is currently a professor of hematology and Program Director of the Medical Laboratory Science program at the University of Alabama, Birmingham. Dr. Josephat has been in the medical laboratory science profession for over 30 years and is board certified by the American Society for Clinical Pathology (ASCP) as a medical laboratory scientist.

Morgan Duke
is currently a senior graduate student in the Medical Laboratory Science program at the University of Alabama, Birmingham. She will be receiving her master’s degree in MLS in the Spring of 2024.