This, that, or both: Platelet aggregation and platelet genetics
Since its invention in the early 1960s, light transmission aggregometry (LTA), also known as platelet aggregation, has played a vital role in the clinical evaluation of bleeding and clotting patients.1 LTA quickly became the reference method or gold standard assay for its ability to detect many inherited, acquired, and drug-induced platelet defects. More recently, in 2016, next generation sequencing (NGS) has significantly expanded the ability for laboratories to diagnose and characterize congenital platelet disorders.2 Massively parallel sequencing technologies, like NGS, have rapidly become the preferred approach for sequencing a large number of genes simultaneously. Unfortunately, neither LTA nor NGS approaches are broadly available across the United States since there are relatively few specialized laboratories offering these methodologies.3 Furthermore, clinicians, and in some circumstances, laboratorians are faced with the dilemma of selecting platelet workups by LTA, NGS, or both. This decision comes with challenges of access, availability, cost, turnaround time differences, and interpretation to name a few. In the orbit of platelet testing, there exists many other assays, including platelet function screens (PFA-100), platelet inhibitory assays, platelet electron microscopy, flow cytometry, lumi-aggregometry, platelet mapping, impedance aggregometry, and many others.
Strengths and challenges of light transmission aggregometry (LTA)
The strength and clinical value of LTA comes from the quick comprehensive assessment of platelet function by activating platelets with approximately 5 to 10 agonists at multiple concentrations. Platelet receptor presence, platelet function, intracellular signaling, shape change, rate of aggregation, primary aggregation, secretion, secondary aggregation, and disaggregation provide key data to correlate to known platelet defect patterns. Platelets are tested directly in a harvested platelet rich plasma (PRP) environment and at standardized platelet concentrations, usually 200 x 109 to 250 x 109 platelets/liter, if the laboratory adheres to Clinical and Laboratory Standards Institute (CLSI) guidelines.4 Agonist-induced platelet responses can also be measured pre- and post-drug to assess the effectiveness of antiplatelet therapy (APT). Certain APT approaches rely on drugs that may be metabolized at varying rates or other approaches when patients may be resistant to a drug’s effect.
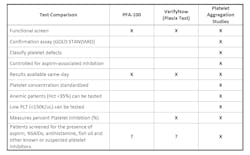
Access to LTA is the largest challenge to obtaining this test. The most recent CAP proficiency testing survey (PF-A 2022) for platelet function listed 162 laboratories that offered LTA in the participation summary. Whole blood drawn for LTA platelet function testing expires in 4 hours or less, making the average of 3.2 capable laboratories per state insufficient for broad utilization. This short sample viability requires that a specialized laboratory be within two hours away to ensure that sufficient time remains to perform the test. Many hospitals and clinics are not fortunate to be in geographic proximity to a specialized coagulation laboratory that performs this test. For that reason, many facilities rely on screening assays, which are summarized in Figure 1. Additionally, the high cost, dedicated instrument, specialty trained laboratorians, clinical interpretation and technical interpretation prevent many hospital laboratories from validating LTA as an on-site service.Strengths and challenges of platelet genetics by NGS
NGS has become a powerful clinical tool to confirm inherited platelet function defects as well as quantitative defects. Generally, these are large panels containing dozens of genes associated with platelet signaling and platelet receptors. These panels are generally thousands of dollars and insurance coverage is important for patients to access the service. Turnaround time for results of platelet genetic panels can vary widely between laboratories, ranging from 2 days to 28 days. The sample required for genetics is generally EDTA whole blood and is very stable when shipped across the nation. Some laboratories are able to accept as little as 1mL of whole blood or buccal swabs to perform the sequencing, making all patient populations suitable to test when clinically justifiable. (Whole blood sample volume requirements ranging from 10 to 20 milliliters may be more than a neonatal patient can safely provide.)
The results of platelet genetic panels are usually several pages long and require an experienced clinician to interpret and correlate clinically. Importantly, the genes and other genetic targets that are included in platelet genetic panels require deep understanding of both clinical and genetic manifestations of platelet disorders; therefore, long term institutional experience in hemostasis and thrombosis is vital in the selection of one laboratory’s panel over another laboratory’s. It is far too easy to wrongly assume that all platelet genetic panels are equivalent. Variants of unknown significant (VUS) are a frequent clinical finding. In those situations, careful assembly of the literature is required to understand the clinical ramifications of a rarely observed variant. Currently, there are only a few laboratories that have built sizable databases of platelet genetics. The public databases are less populated with platelet genetic data, which further complicates interpretation. Similar to LTA, NGS requires the purchase of expensive sequences that require a dedicated scientific staff to maintain and operate. Molecular biology trained PhD scientists are the most common staff to support NGS services; however, specialized clinical laboratory scientists may also support NGS services.
Clinical situations where both tests are warranted
Several clinical situations warrant consideration of ordering both LTA and NGS workups. These include antiplatelet therapy, confirmation of congenital platelet disorders, dysfibrinogenemia suspected, severe bleeding in a pediatric patient, liver disease, and patients with low platelet counts. For example, antiplatelet therapy often prevents clinicians from removing therapy to measure native platelet function. Additionally, observed defect patterns in LTA require genetic confirmation to identify causative mutations.5, 6
In conclusion, LTA and NGS platelet tests provide comprehensive functional and genetic options for working up patients suspected of having an acquired or inherited defect.7 These are very specialized tests that require a specialty-trained scientist to perform. Hospitals and clinicians need to carefully consider the selection of NGS platelet genetic services as panel designs vary across the United States.
References
- Born GV. Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature. 1962;194:927-929. doi:10.1038/194927b0.
- Lentaigne C, Freson K, Laffan MA, Turro E, Ouwehand WH, BRIDGE-BPD Consortium and the ThromboGenomics Consortium. Inherited platelet disorders: towards DNA-based diagnosis. Blood. 2016;127(33):2814-2823. doi:10.1182/blood-2016-03-378588.
- Beck TF, et al. Systematic Evaluation of Sanger Validation of NextGen Sequencing Variants. Clin Chem. 2016 April;62(4):647-654.
- Clinical and Laboratory Standards Institute (CLSI). Platelet Function Testing by Aggregometry: Approved Guideline. CLSI document H58-A. Clinical and Laboratory Standards Institute, 940 West Valley Road, Suite 1400, Wayne, Pennsylvania 19087-1898 USA, 2008.
- Hayward, C, et al. Development of North American consensus guidelines for medical laboratories that perform and interpret platelet function testing using light transmission aggregometry. Am J Clin Pathol 2010;134:955-963. doi:10.1309/AJCP9V3RRVNZMKDS.
- Kottke-Marchant K, Corcoran G. The laboratory diagnosis of platelet disorders. Arch Pathol Lab Med. 2002;126(2):133-146. doi:10.5858/2002-126-0133-TLDOPD.
- Guidelines on platelet function testing: The British Society for Haematology. BCSH Haemostasis and Thrombosis Task Force. J Clin Pathol. 1988;41: 1322-1330. doi:10.1136/jcp.41.12.1322.
About the Author
Mike Ero MT, CLS, MBA
is the CEO and founder (2003) of Machaon Diagnostics, headquartered in Berkeley, CA with a second CAP/CLIA site in New Orleans, LA. He has built a solutions-based, team approach to laboratory medicine, designing new and innovative laboratory strategies to meet unmet needs in the healthcare and bioscience industries. He is a laboratory expert and CAP inspector with over 25 years of healthcare experience in the areas of coagulation, platelets, complement, genetics, and rare disease.