Blood clots can kill: A current overview of thrombosis risk factors
Hellen Keller famously wrote, “Life is either a daring adventure or nothing at all.” Certainly, with respect to blood flow consistently pushing throughout the body to maintain living functions, constantly changing conditions lead to a constantly risky endeavor. The need continues 24 hours per day, 7 days per week, 365 days per year, with health problems either in an acute or chronic sense, arising, even in otherwise healthy individuals.
The public at large is not fully aware of the signs and symptoms of venous thromboembolism (VTE), also known as thrombosis. A significant public health problem even before the COVID-19 pandemic, 274 people die every day as a result of blood clots just in the U.S. alone, with 100,000-300,000 deaths occurring each year, greater than the total number of people dying annually from AIDS, breast cancer and car crashes combined, according to data from the Centers for Disease Control & Prevention (CDC). Two thirds of cases occur in outpatients, with diagnostic and prescription costs between $7,594-$16,644 per patient, contributing well over $2 billion in total cost to the healthcare system annually.1 Even if a patient survives a bout of PE or DVT, post thrombotic syndrome (PTS) occurs in up to 4-10% of these patients, causing venous insufficiency, inflammation, venous hypertension, and resulting damage to venous valves.
The level of knowledge around VTE among patients and providers could be greatly strengthened. Confusion exists even among emergency medicine providers, who may feel they cannot rely on the D-dimer assay, since they may see it more as a biomarker often elevated, especially in elderly, cancer, or obstetric patients. Public awareness of the dangers of VTE is also low, and people may not recognize the signs and symptoms, including chest pain, which gets worse as you inhale, until it is too late, and the blood clot has negatively impacted lung function.
VTE includes dangerous cases of pulmonary embolism (PE), or blood clots in the lungs; or deep venous thrombosis (DVT), often found in lower extremities, especially the lower venous system. A major contributor to morbidity and mortality, PE and DVT significantly impact the overall healthcare system, but opportunities currently exist to properly use laboratory diagnostic and clinical algorithmic tools to conserve healthcare spending while also optimizing patient outcomes.
Rudolph Virchow, a pathologist in the mid-1800s, initially characterized the VTE disease state along with many of the risk factors, publishing a description of blood clots in 1859, which he called “Embolia.” The underlying risk factors of VTE were later categorized by other clinical scientists into Virchow’s triad, with three different categories: (1) circulatory stasis, or overall blood movement; (2) vascular wall injury, including trauma or surgery; and (3) hypercoagulable state, such as malignancy, but also pregnancy and the peripartum period.
Importance of the D-dimer test
The COVID-19 pandemic has put a spotlight on venous and arterial thrombotic risk due to the viral disease arising from SARS-CoV-2 resulting in increased thrombotic risk. Individual patients can develop VTE in many sites throughout the body, contributing to the mortality and morbidity picture of COVID-19. Laboratory tests not only help clinicians with diagnosis and staging of viral load, but by use of the automated D-dimer test, mostly available on demand as a routine assay, providers will be able to glean information on potential patient outcomes, depending on the result. Indeed, the recently updated guideline from the International Society on Thrombosis and Haemostasis (ISTH) outlines the importance of D-dimer to identify potential thrombotic issues in COVID-19 patients presenting to the intensive care unit (ICU). According to the ISTH guideline, COVID-19 patients exhibit not only D-dimer elevations, but also elevated fibrinogen and factor VIII, and shortened activated partial thromboplastin time (aPTT).
Published studies have consistently shown COVID-19 patients with elevated D-dimer (> 4.0 μg/mL) have poor overall outcomes, but confirmation of VTE in these patients is difficult due to patient instability or the protocol of a prone position in acutely ill patients. Regardless, use of the IMPROVE VTE score, a clinical algorithm intended for VTE identification in hospitalized patients, along with D-dimer levels > 2 times upper limit of normal (ULN) identified patients with greatly increased VTE risk, who could benefit from extended duration prophylactic anticoagulation.2 Thus, the D-dimer assay is useful not only to exclude VTE presence in suspected patients, but also for monitoring ongoing status in at-risk, critically ill patients with COVID-19.
The use of D-dimer in COVID-19 in hospitalized COVID-19 patients reflects its well-established use in hospitalized DIC patients, along with other fibrin related markers, decreased fibrinogen, decreased platelet count, and increased prothrombin time (PT).3 Adding to the COVID-19 laboratory testing picture, individuals suspected of Thrombosis with Thrombocytopenia Syndrome (TTS), have sometimes been observed in individuals receiving the COVID-19 vaccine up to 4-28 days prior to presentation. Those patients also show D-dimer elevations, along with changes in platelet count, aPTT, PT, fibrinogen, with confirmation using separate heparin-induced thrombocytopenia (HIT) assays.4
Going back to use of D-dimer in non-COVID-19 related VTE, automated high sensitivity immunoturbidimetric D-dimer assays are used in concert with a pretest probability (PTP) score (e.g., Wells Score, Geneva Score, YEARS Algorithm). D-dimer is an antigenic fragment reflective of recent fibrinolytic activation and fibrin clot presence in the plasma with the value of the assay lying in its high negative predictive value (NPV) to rule out PE and DVT. With relatively nonspecific symptoms, the test aids clinical decision making for clinicians examining patients presenting in emergency departments (ED) and urgent care facilities.
D-dimer tests available commercially are usually validated with a cutoff value of 0.50 µg/mL Fibrinogen Equivalent Units (FEU) for exclusion of PE or DVT, in concert with the PTP. Some available assays report in different units, such as ng/mL, or even in units corresponding to different defined breakdown products, D-dimer units (DDU). Alternative cutoffs may need to be considered for D-dimer use in other acute illnesses, including COVID-19 and disseminated intravascular coagulation (DIC), but institutions rarely report out validated results other than for VTE exclusion.
Clinical algorithmic tools
Various PTP scores have been established and validated in large clinical trials relating patient symptomology to outcomes, and include the Wells Score, Geneva Score, and YEARS algorithm. The different algorithms have slightly different criteria, but providers in North America generally use the Wells Score. Both clinical guidelines and assay platform manufacturers recommend utilization of a PTP in concert with a D-dimer test. The Wells Score can be programmed into the hospital information system as part of a required procedure when ordering laboratory D-dimer, establishing clinical history including active cancer, localized tenderness, recent paralysis or bedridden status, leg swelling, elevated heart rate, hemoptysis, and previous DVT/PE. In a real-world sense, other environmental or lifestyle, dietary (e.g., supplements), health, and genetic factors are important to overall thrombosis or bleeding risk, but the Wells Score depends on clinical history presented to the ordering physician at the bedside. Depending on the outcome of the score, either a D-dimer assay is ordered to exclude PE or DVT, if the patient is found low or moderate risk, or for high-risk patients, the patient is sent directly to confirmatory imaging or ultrasound to establish presence of the clot.
Risk factors for VTE progression
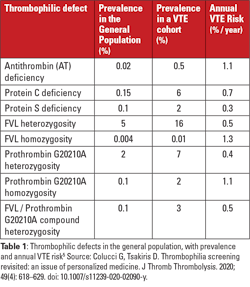
Underlying inherited genetic mutations related to VTE progression include factor V Leiden (FVL), prothrombin gene mutation G20210A (PGM), deficiencies of antithrombin, protein C, or protein S. Associated prevalence and annualized VTE risks for the associated conditions potentially seen by patients visiting hospitals are described here (see Table 1).5 In addition, fibrinolytic factors, though less recognized to play a role from a laboratory or provider perspective, can lead to deficiencies of fibrinolytic function, which then serve to downregulate clot breakdown, extending clot appearance in the plasma, resulting in VTE progression, and potential arterial thrombosis. Those inherited aspects of thrombotic risk are combined with acquired risk factors including advanced age, previous VTE history, cancer, obesity, and/or antiphospholipid antibody presence (leading to antiphospholipid syndrome), increasing overall thrombosis risk.
These single or multiple hits, when combined with triggering events, such as estrogen therapy or oral contraception, pregnancy, surgery, or immobilization, will take the patient across the positive threshold, resulting in DVT or PE, or arterial thrombosis leading to transient ischemic attack (TIA), stroke, or heart attack.
Analysis of Medicare records in post-menopausal women showed overall post-VTE mortality was 8% at 28 days, and 22% at 1 year, largely driven by underlying comorbidities. In addition, African American women analyzed in the study showed higher VTE incidence compared to other populations, which was consistent with other studies.6
Overutilization of imaging studies
To examine clinician practices around D-dimer testing utilization, while simultaneously assessing the population health impact of CTPA overutilization across large health systems with varied site sizes and patient populations, a large cross-sectional analysis of ED patients in 27 different hospitals who previously underwent PE diagnostic imaging. Takeaway points from the study included the following:
- Institutional use of imaging procedures vary widely across different hospital sites, but a focus on quality improvement can improve CTPA yield rates.
- Due to higher utilization in women and at the ages shown in the study, on a population basis, they are at more risk from radiation exposure from CTPA procedures.
- D-dimer and PTP use directly correlates to improved PE yield rates, showing the important role of D-dimer at reducing unnecessary imaging procedures.7
The findings have clear implications for proper utilization of D-dimer testing across the healthcare system, and the findings will inform laboratory staff interested in developing local best practices. Given the study findings, clinician uncertainty around D-dimer testing should reduce once recognition of the importance of D-dimer in the care pathway are recognized.
Instead of following the PTP score and using D-dimer assays in patients with low to moderate probability to risk stratify patients, clinicians often skip the D-dimer and go directly to imaging, or simply run D-dimer and confirmatory imaging in all suspected patients, leading to unnecessary imaging procedures. Overuse of expensive and time-consuming imaging procedures, such as computed tomography pulmonary angiography (CTPA) is associated with significant population cancer risk from exposure to radiation. In addition, patients sometimes experience unexpected kidney injury from contrast nephropathy, and ambiguous or false-positive imaging results complicate management.
CTPA utilization has shown overall increases in U.S. hospitals, while concomitant PE diagnostic yield or the effectiveness of confirming diagnosis by CTPA has fallen, most likely due to improper utilization of D-dimer and PTP.8 Indeed, a recent publication summarizing interviews with 23 clinical providers in two states indicated main barriers to proper utilization of imaging techniques included limited use, distrust, or lack of knowledge on use of institutional-based clinical decision support (CDS) tools, such as use of Wells Score and D-dimer testing.9 Further, in the emergency department (ED) setting, approximately 5% of all patients have a test for VTE, and the median age of persons tested for PE in the ED is 46-years-old. Radiation exposure presents a significant risk later in life, especially in the case of women, but the PE positivity rate for patients tested is less than 5%, so given the increased risk inherent in the procedure, the PE positivity rate is low.10
Treatment of VTE is commonly performed by prescribing anticoagulants, provided either parenterally (intravenous or subcutaneous) for hospitalized patients, or in oral form in outpatients. Many parenteral and oral anticoagulant choices are now available for patients and providers to customize therapy, so treatment is greatly accessible with well-known safety outcomes.
On the bright side, not including the impact from the pandemic, overall trends had appeared positive with regards to PE incidence and outcomes in the United States, with one study of approximately 810,000 Medicare patients over 65-years-old, from 1999-2015 with a PE as their principal condition on discharge, showing decreases in case fatality rates from 8.7% to 4.0% and decreased adjusted 30-day case fatality rate from 12.7% to 9.4%, along with a decreased hospitalization rate.11 Thus, PE care trends have been favorable, due to increased physician education and spreading of best practices across integrated delivery networks (IDNs). In any event, laboratorians must keep their colleagues on front lines of care informed to make sure the importance of VTE prevention is well understood, and the role of the lab D-dimer assay is clearly established.
Note:
To ensure patients and the public increase their level of understanding of the dangers of VTE, the International Society on Thrombosis and Haemostasis (ISTH) started an annual education and awareness day called World Thrombosis Day (WTD), taking place each year on October 13.
References
- Beckman MG, Hooper WC, Critchley SE, Ortel TL. Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38(4 Suppl):S495-501. doi: 10.1016/j.amepre.2009.12.017.
- Spyropoulos AC, Levy JH, Ageno W, Connors JM, Hunt BJ, Iba T, Levi M, Samama CM, Thachil J, Giannis D, Douketis JD. Subcommittee on Perioperative, Critical Care Thrombosis, Haemostasis of the Scientific, Standardization Committee of the International Society on Thrombosis and Haemostasis. Scientific and Standardization Committee communication: Clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020 Aug;18(8):1859-1865.
- Taylor FB Jr, Toh CH, Hoots WK, Wada H, Levi M; Scientific Subcommittee on Disseminated Intravascular Coagulation (DIC) of the International Society on Thrombosis and Haemostasis (ISTH). Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001 Nov;86(5):1327-30.
- Nazy I, Sachs UJ, Arnold DM, et al. Recommendations for the clinical and laboratory diagnosis of VITT against COVID-19: Communication from the ISTH SSC Subcommittee on Platelet Immunology. J Thromb Haemost. 2021;19(6):1585-1588. doi: 10.1111/jth.15341.
- Colucci G, Tsakiris DA. Thrombophilia screening revisited: an issue of personalized medicine. J Thromb Thrombolysis. 2020;49(4):618-629. doi: 10.1007/s11239-020-02090-y.
- Burwen DR, Wu C, Cirillo D, Rossouw JE, Margolis KL, Limacher M, et al. Venous thromboembolism incidence, recurrence, and mortality based on Women’s Health Initiative data and Medicare claims. Thromb Res. 2017; 150: 78-85. doi: 10.1016/j.thromres.2016.11.015.
- Kline JA, Garrett JS, Sarmiento EJ, Strachan CC, Courtney DM. Over-testing for suspected pulmonary embolism in American emergency departments: the continuing epidemic. Circ Cardiovasc Qual Outcomes. 2020;13:e005753. doi: 10.1161/CIRCOUTCOMES.119.005753.
- Venkatesh AK, et al. Trends and variation in the utilization and diagnostic yield of chest imaging for Medicare patients with suspected pulmonary embolism in the emergency department. AJR Am J Roentgenol. 2018 Mar;210(3):572-577. doi: 10.2214/AJR.17.18586.
- Westafer LM, et al. Provider perspectives on the use of evidence-based risk stratification tools in the evaluation of pulmonary embolism: a qualitative study. Acad Emerg Med. 2020 Jun;27(6):447-456. doi: 10.1111/acem.13908.
- Feng LB, Pines JM, Yusuf HR, Grosse SD. U.S. trends in computed tomography use and diagnoses in emergency department visits by patients with symptoms suggestive of pulmonary embolism, 2001-2009. Acad Emerg Med. 2013 Oct;20(10):1033-40. doi: 10.1111/acem.12221.
- Bikdeli B, Wang Y, Jimenez D, Parikh SA, Monreal M, Goldhaber SZ, Krumholz HM. Pulmonary embolism hospitalization, readmission, and mortality rates in US older adults, 1999-2015. JAMA. 2019 Aug 13;322(6):574-576. doi: 10.1001/.jama.2019.8594.
About the Author

Paul Riley, PhD, MBA
serves as Scientific Business Development Manager at Diagnostica Stago, Inc. Paul earned a PhD in biochemistry from Temple University in 2006, with the subject of the dissertation regarding the function of coagulation factor XI. He also did postdoctoral training in a related area before becoming a product manager and scientific affairs specialist in 2009. During his time at Stago, Paul also completed an MBA degree program at Cornell University in 2014. He has spoken to dozens of medical technologist, clinical laboratory scientist, pharmacist, and clinician audiences about various topics within hemostasis and coagulation.