Pandemic care: The need for hemostasis testing in critically ill COVID-19 patients
The etymology of the word pandemic arises from the Greek pándēmos, meaning “common” and “public.” Almost all people worldwide are now dealing in some way with the pandemic resulting from the common, and publicly spread, SARS-CoV-2 virus infection. Some individuals have the corresponding respiratory and inflammatory disease, now known as COVID-19. The name originates from the capitalized letters shown: COrona VIrus Disease, with the outbreak first observed in December 2019.
The article will describe hemostasis-related changes observed in COVID-19 patients, especially those with acute respiratory distress syndrome (ARDS) and various stages of disseminated intravascular coagulation (DIC).
Writing during the sixth week of my community’s quarantine, with deaths from the illness still on the upswing, but working from home and managing homeschooling with my family, we constantly receive information via social and traditional media about COVID-19. We only rarely hear in the media about the risk of venous thromboembolism (VTE) in these patients or associated disseminated intravascular coagulation (DIC). More often, we hear about the symptoms and problems associated with ARDS related to COVID-19, which has been at the forefront of urgent clinical-management needs, requiring rapid development of best practices and taking on crisis proportions in many hotspots around the world.
The balance of hemostasis includes the need to activate the coagulation pathway when necessary, but also to regulate the coagulation pathway to prevent unwanted clot formation, playing a critical role in maintaining blood fluidity for otherwise healthy individuals. In critically ill patients, including those with COVID-19, the balance often falls to one side or another, leading to either bleeding or thrombotic complications.
Hidden sign of trouble
Currently, patients treated in hospitals for COVID-19 are experiencing a tendency toward inflammatory response and resulting hypercoagulability, a broad term referring to a generalized risk of developing pathological blood clots, also known as thrombosis, or venous thromboembolism (VTE). Emergency physicians routinely assess VTE risk when a patient presents to the emergency department with clinical signs or symptoms suggesting a potential case of pulmonary embolism (PE), or thrombosis in the lungs. PE often follows an occurrence of deep vein thrombosis (DVT), thrombosis in the leg veins, usually in the space immediately in the outflow space behind a venous valve where blood flow rates are lower. Risks for VTE would include recent trauma, active cancer, recent surgery and immobility. Clinical signs of VTE would include, but are not limited to, increased heart rate, difficulty breathing (with or without extremity swelling) and tenderness.
Commonly, clinicians order automated D-dimer assays for patients to rule out VTE, but also to assess patients suspected of consumptive coagulopathies, such as DIC. Inpatients may have increased risk for VTE from hospital-related issues (e.g. immobility), as well as primary clinical events, including trauma, cancer treatment and/or viral infections such as COVID-19. Adding the coagulation imbalances to the mix of other medical complications can produce a life-altering thrombosis, stroke, severe bleeding and/or death.
A challenging chronic condition
In addition, length of anticoagulant prophylaxis will be different among patients, depending also on their primary condition, length of stay, comorbidities and potential medication incompatibilities. Clinicians most commonly give anticoagulation for COVID-19 patients by infusion of intravenous unfractionated heparin (UFH), or low molecular weight heparin (LMWH), which is given intravenously or subcutaneously. Importantly, prophylactic anticoagulation is required due to high incidence of thrombosis in COVID-19 patients, with Cui, et al. finding VTE incidence of approximately 25 percent, with 9.9 percent (8/81) mortality outcome.4
Early symptoms associated with symptomatic COVID-19 include fever, cough, shortness of breath, loss of taste and smell, along with sore throat and other symptoms associated with other communicable respiratory illnesses (such as influenza, tuberculosis, Group A streptococcus, Haemophilus influenzae type b, among others). While public health professionals continue to battle against the pandemic by providing recommendations and limiting the spread by testing and tracing individuals with the COVID-19 virus, healthcare practitioners face formidable challenges to not only manage ARDS in the patients, but also recognize and treat the secondary thrombotic disorders as well.
Laboratory markers of hypercoagulability
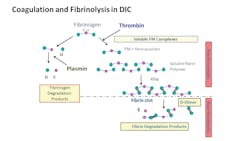
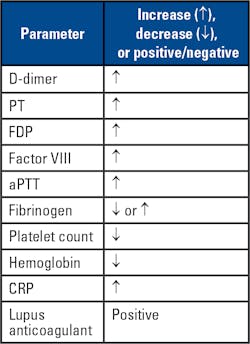
In COVID-19 patients, VTE arises from cascading cytokine activation, creating a storm of inflammation and resulting in thromboses within large and small vasculature of various severities and locations disseminated throughout the body. Markers of hypercoagulability, including significant increases in D-dimer and (FDP) levels, prolongation of prothrombin time (PT) and activated partial thromboplastin time (aPTT) and decreased fibrinogen levels are all observed in COVID-19 patients.5
In a recently published study by Tang, et al. from China, 71 percent of COVID-19 patients experienced DIC, with all showing increased D-dimer, PT, aPTT, and decreased fibrinogen levels.5 In the Tang, et al. study, D-dimer levels, usually > 1000 ng/mL D-dimer (fibrinogen equivalent units, FEU) directly correlated with severity of the patient outcomes,6 though the findings will require further studies. Interestingly the PT, D-dimer and FDP results were predictive of mortality outcomes at baseline, but the other parameters reported were predictive later in the disease progression. In addition, patient samples may also show positive results for lupus anticoagulant (LA) assays, including dilute Russell Viper Venom time (dRVVT), and hexagonal phase phospholipid neutralization assays.7 Positive LA results are found transiently in other patient populations with acute viral and other illnesses and may not reflect active thrombosis in COVID-19 patients.
Other acute-phase markers show systematic increases, including C-reactive protein (CRP), an interfering substance in most aPTT-based LA assays. Other coagulation proteins with potential abnormalities in DIC and inflammatory states could include protein C (PC), protein S (PS) and antithrombin (AT), shown to be lower in these patients, the result of rapid consumption, similar to the rapid consumption of procoagulant zymogens of the coagulation cascade. Results for the aforementioned parameters will only be informative when the samples are collected during the acute phase. Hematological changes in the patients observed in China also include leukocytosis and thrombocytopenia,7 with observed changes from the breaking studies described shown in the table accompanying the article.
In patients with elevated baseline aPTTs, the chromogenic anti-Xa assay will be the preferred monitoring test for patients receiving UFH, per the institution’s selected target ranges for prophylactic or thrombosis treatment dosing. LMWH monitoring using anti-Xa should also be considered in the COVID-19 patients, especially if there are renal clearance issues. For anti-Xa monitoring, reagent kits without added AT are preferable to accurately determine the physiologic response to coagulation. UFH resistance has been reported, potentially due to decreases in AT levels, or increases in factor VIII (FVIII) or fibrinogen in the patients with overt, or chronic DIC in COVID-19.8 If the UFH resistance is caused by decreased AT, then an anti-Xa assay kit with added AT may not reflect the in vivo therapeutic response.9 With bleeding risk significantly elevated for many of the COVID-19 patients, alveolar bleeding could be a serious complication, and not all patients will require anticoagulation.
Conclusions
Public health professionals continue to fight the disease spread in close cooperation with hospital systems. The public’s continued cooperation in social distancing will continue to decrease incidence, and with attention to the thrombotic issues described here, clinicians can work to ensure the best possible patient outcomes. Even in the best possible care scenario, the burden of thrombosis will be a significant contribution to the overall mortality picture associated with COVID-19, making awareness of thrombosis by clinicians and the public critical to combat the overall impact.
References:
- Levi M. Pathogenesis and diagnosis of disseminated intravascular coagulation. Int J Lab Hematol. 2018 May;40 Suppl 1:15-20.
- Boral BM, Williams DJ, Boral LI. Disseminated Intravascular Coagulation. Am J Clin Pathol. 2016 Dec;146(6):670-680.
- Soundar EP, Jariwala P, Nguyen TC, Eldin KW, Teruya J. Evaluation of the International Society on Thrombosis and Haemostasis and institutional diagnostic criteria of disseminated intravascular coagulation in pediatric patients. Am J Clin Pathol. 2013 Jun;139(6):812-6.
- Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020 Apr 9. doi: 10.1111/jth.14830. [Epub ahead of print]
- Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020 Apr;18(4):844-847.
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 Mar 28;395(10229):1054-1062.
- Zhang Y, Xiao M, Zhang S, et al. Coagulopathy and anti-phospholipid antibodies in patients with COVID-19. N Engl J Med. 2020 Apr 23;382(17):e38.
- Beun R, Kusadasi N, Sikma M, Westerink J, Huisman A, et al. Thromboembolic events and apparent heparin resistance in patients infected with SARS-CoV-2. Int J Lab Hematol. 2020 Apr 20. doi: 10.1111/ijlh.13230. [Epub ahead of print]
- Gehrie E, Laposata M. Test of the month: The chromogenic antifactor Xa assay. Am J Hematol. 2012 Feb;87(2):194-6.
About the Author

Paul Riley, PhD, MBA
serves as Scientific Business Development Manager at Diagnostica Stago, Inc. Paul earned a PhD in biochemistry from Temple University in 2006, with the subject of the dissertation regarding the function of coagulation factor XI. He also did postdoctoral training in a related area before becoming a product manager and scientific affairs specialist in 2009. During his time at Stago, Paul also completed an MBA degree program at Cornell University in 2014. He has spoken to dozens of medical technologist, clinical laboratory scientist, pharmacist, and clinician audiences about various topics within hemostasis and coagulation.