Extend the power of HPV testing
BD Onclarity HPV Assay is an FDA-approved test with extended genotyping to individually identify high-risk HPV types beyond 16, 18 and 45, providing a more specific risk assessment for an extended set of individual HPV types, and enabling tracking of those types over time (persistence). Reagents are stored at room temperature, making consumable loading easier.
FDA-cleared & categorized, POC fentanyl test
Carolina Liquid Chemistries introduces the Fentanyl Urine Detection Test on CLC’s next-generation RYAN immunofluorescent analyzer. This FDA cleared and CLIA categorized, moderately complex, compact, point-of-care system detects fentanyl in human urine at a cutoff concentration of 1.0 ng/mL in <6 minutes, produces a qualitative, chartable result, and is interfaceable to LIS.
Addressing the challenges of immunofluorescence testing
The UNIQO 160 (model no. YG 2900-0101) automates all steps of immunofluorescence testing for up to 160 samples within one device. The UNIQO 160 supports ANA, ANCA, and dsDNA protocols, executes up to 18 workflows in a single run, and includes EUROLabOffice 4.0 software, enabling communications with LIS networks.
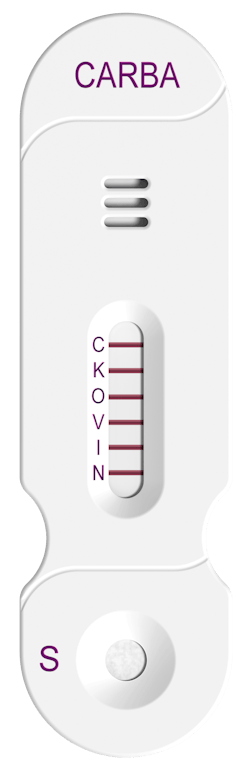
Detect the “Big 5” in 15 minutes
NG-Test CARBA 5 is a rapid, multiplex, phenotypic, FDA cleared test capable of detecting KPC, OXA-48-like, VIM, IMP and NDM carbapenemases produced by Enterobacterales and Pseudomonas aeruginosa. CARBA 5 is a lateral flow assay that detects the gene expression, which is crucial to aiding therapeutic decisions. Results in 15 minutes.
Urine analyzer
The Evidence MultiSTAT DOA Urine MultiPlex is intended for prescription use with the Evidence MultiSTAT. Screening for methamphetamine, noroxycodone, benzodiazepines I, methadone, phenobarbital, tramadol, phencyclidine, buprenorphine and 6-acetylmorphine. The MultiSTAT is a fully automated analyzer for the qualitative determination of parent drug molecule and metabolites of drugs in human urine.
Simplified newborn screening for SMA, SCID
The EONIS Q system is a CE-IVD declared platform enabling laboratories to adopt molecular testing for SMA and SCID in newborns. The EONIS Q system simplifies and streamlines molecular testing with an innovative workflow, inclusive of the EONIS Q96 instrument, the EONIS SCID-SMA kit and dedicated EONIS EASI software.
AMH information guides in vitro fertilization decisions
Siemens Healthineers now offers the Anti-Müllerian Hormone (AMH) Assay to quickly evaluate ovarian reserve. The AMH Assay is an important addition to a laboratory's reproductive endocrinology test menu as it aids a physician's initial assessment about initiating in vitro fertilization with a patient. Physicians use AMH test results, which indicate the volume of remaining eggs, to determine whether a patient's ovaries may respond favorably to IVF.
Automation-friendly performance in MDx testing
The Applied Biosystems TaqPath DuraPlex 1-Step RT-qPCR Master Mix is an automation-friendly, single-tube reagent optimized for highly sensitive pathogen detection even in the presence of inhibitors. This benchtop-stable formulation can multiplex 6-targets in a single well and is approved for use in laboratories running or developing high-throughput molecular diagnostic assays.
Introducing syphilis serology proficiency testing – Waived methods
WSLH PT introduces the Syphilis Serology proficiency testing program for waived methods for 2024. This program is intended for use with Syphilis Health Check and includes three, 1ml liquid serum samples shipped twice per year.
Reliable testing solution for EBV infections
Make Sebia Autoimmune & Infectious Diseases your first choice for high-performing, easy-to-use testing solutions for Epstein-Barr virus (EBV) infections. ZEUS ELISA EBV Test Systems are an ideal solution for sensitive, reliable detection of antibodies to EBV antigens. Our common reagents and universal protocol enable optimized workflow efficiency.