And they continue to grapple with supply shortages, particularly for items involving plastic, such as test tubes. Test kits for some non-COVID-19 pathogens also are difficult to get.
At the peak of the COVID-19 pandemic last winter, the company tested 8,000 specimens a day for SARS-CoV-2 at its lab in Dayton. It now tests less than 100 samples a day. “Half of my clients test for COVID, and their stories are incredibly similar,” Ingle said. “I have never seen a more dramatic drop in anything in my entire life.”
Medical City Dallas also has seen a drop in volume — although not quite as spectacularly. It performs all SARS-CoV-2 testing in Texas on behalf of its parent company, Hospital Corporation of America (HCA). At Medical City Dallas’ molecular lab, COVID-19 testing volumes have dropped from 700-800 tests a day in November 2020 to 350-400 per day in May 2021.
Positivity rates also are declining for HCA’s patients in Texas. The rate of positivity peaked at about 30% and now is at about 5%, says Harvey Jones, MS, MPH, C(ASCP) SC, Director of Laboratory Services at Medical City Dallas.
Medical City Dallas and Clinical Lab Consulting are not alone; many labs are adapting their testing strategies to fit the current phase of the pandemic.
To find out how labs are approaching diagnostics for COVID-19 and other infectious and chronic diseases, Medical Laboratory Observer queried readers through a State of the Industry (SOI) survey. Conducted in April and May 2021, it is the third of four SOI surveys and associated articles that MLO plans to release in 2021.
Most of the survey respondents work in lab administration. The breakdown was 54% who identified themselves as lab managers, administrators, or supervisors; 21% as lab directors; and 5% as section heads or department managers. Nearly 58% of them work in hospital labs, compared with 13% in independent labs, 10% in physician’s office labs, 7% in government labs, and 6% in group practice labs. The remander work in other settings.
Survey respondents were distributed across different sizes of labs. Thirty percent work at labs with 1-10 employees, 13% at labs with 11-20 employees, 15% at labs with 21-50 employees, 21% at labs with 51-100 employees, and 20% at labs with more than 100 employees.
COVID-19 Testing
As might be expected, the use of multiple analyzers was more common with larger organizations than smaller ones, the survey found. Similarly, hospital labs comprised the bulk of all locations utilizing three or more analyzers.
During the peak of the pandemic, five-hospital Monument Health also relied on an algorithm the lab team developed to prioritize which samples to process in-house, such as those from high-risk patients or staff members. Other specimens could be sent to reference labs, if necessary.
Now that testing volume is decreasing, Monument Health, like other organizations, is deploying different strategies. It is relying more heavily on rapid molecular tests at the point of care, rather than sending samples via courier to the main lab, where they would be processed on a high-throughput platform.
During the peak, testing demand was so high that the system’s outpatient locations simply could not keep up with the volume using the rapid testing method, which requires providers to process samples within an hour, DeMenge explains.
The system operated drive-thru testing sites and processed more than 5,000 samples per week during the peak, using five different platforms.
Testing for SARS-CoV-2 is not necessarily a break-even endeavor, according to respondents to MLO’s State of the Industry survey. A total of 28% said reimbursement for SARS-CoV-2 was covering their costs, while 16% they were not covering their costs; another 36% were not sure. A total of 20% said the question did not apply to them.
Testing volumes for inpatients with COVID-19 also have declined throughout the United States. There are simply fewer of them to test for medical issues associated with COVID-19, such as acute respiratory distress syndrome (ARDs), shock, and organ failure.
For example, Medical City Dallas had five inpatient units dedicated to COVID-19 patients during a surge in the pandemic from December 2020 through January 2021. At that time, the staff was treating as many as 110 COVID-19 patients concurrently. The hospital has shut four of those units down, leaving space at one unit available if it admits patients with COVID-19 in the future.
When they do have patients with COVID-19, Medical City Dallas and other hospitals process a variety of tests to assess the medical status of COVID-19 inpatients.
Among MLO’s survey respondents, the percentage of them who conduct these types of tests was 53% for D-dimer, 50% for C-reactive protein, 30% for fibrinogen, 10% for IL-6, 6% for FDP and 4% for cystatin C (CysC).
Preparing for flu season
However, Jones expects to manage those assays in-house during the upcoming season. The organization has a variety of syndromic testing options, including a multiplex panel with 22 biomarkers, which is primarily used for very ill pediatric patients at Medical City Children’s Hospital.
Monument Health reserves its large, expensive panels for critically ill patients. Physicians treating other patients choose from a combination test for COVID-19/ flu AB/RSV, or single-pathogen assays for each of those viruses.
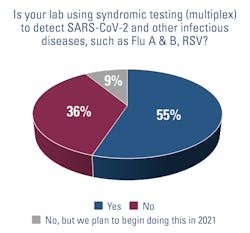
More than half of respondents to the State of the Industry survey (55%) also use syndromic testing to detect SARS-COV-2 and other respiratory diseases; another 9% said they plan to begin offering it in 2021.
Serology testing
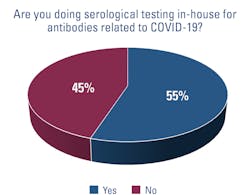
HCA, including Medical City Dallas, opted not to ramp up serology testing in-house. “If there’s a need, we send it out,” Jones says, adding that there has been very little demand for the test from the hospital’s physicians.
The situation is just the opposite in New Jersey, where CentraState Healthcare System has been processing qualitative serology tests since May 2020. It is in the process of validating a quantitative test, and Pacetti regularly fields queries from patients who ask when the new test will be available.
The problem is that patients who are fully vaccinated want to find out if they are “immune” to SARS-CoV-2, Pacetti explains, but the new quantitative test will not be able to provide them with that level of comfort. They will simply find out if they have antibodies. “It’s a very slippery slope on how you report it. The physicians can order it, but we want to make sure they interpret it correctly.” Indeed, she says, the lab’s medical director has been providing information on test-result interpretation to providers in advance of the new product’s debut at the health system.
The U.S. Food and Drug Administration (FDA) issued a similar message in an announcement about using serology testing. “Antibody tests can play an important role in identifying individuals who may have been exposed to the SARS-CoV-2 virus and may have developed an adaptive immune response. However, antibody tests should not be used at this time to determine immunity or protection against COVID-19 at any time, and especially after a person has received a COVID-19 vaccination,” the FDA said.1
Managing staff during COVID-19
Although always a challenge, lab managers struggled to find enough staff members to cover testing demands during the pandemic’s peaks.
At Medical City Dallas, staffing issues were caused by numerous factors, such as a surge in employees who retired, combined with those who found new jobs while they were on furlough from the hospital. Still, other staff members could not work, because they were at home supervising their children while schools were closed.
“We’re back to regular staffing now, but it took us the rest of the year to recover,” Jones said.
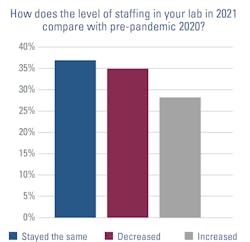
But other labs — 35% of survey respondents — are not as fortunate; they have fewer staff members than they did in 2020, while 37% have the same amount and 28 percent have more.
Supply chain issues
Other vestiges of the pandemic’s onslaught remain, particularly in the supply chain.
As diagnostic companies focused on producing supplies for SARS-CoV-2 testing, they often scaled back production of other items that use the same manufacturing lines.
For example, Monument Health struggles to get test kits for chlamydia and gonorrhea, so it reserves the small number of test kits that it has in stock from its usual source for the emergency department. It also has sourced kits to run on another vendor’s platform as a secondary solution. But because it is a high-throughput analyzer, and the hospital’s volume of tests is small, using it less cost effective, DeMenge explained.
For other outpatients — about 2 or 3 per day — the lab has gone “back to more to traditional lab work,” specifically, a wet prep, DeMenge said. In this situation, the lab advises physicians to combine the results from a wet prep, which is less sensitive than PCR, with their clinical judgement, based on a patient’s symptoms.
CentraState Healthcare System also has grappled with shortages; most recently for the blue-top test tubes used for coagulation studies, such as for PT/INR and PTT tests, which measure blood-clotting rates. “We go through 200 a day,” Pacetti says.
To manage the shortage, CentraState Healthcare System is reserving the bulk of its supply for the critically ill, such as inpatients with COVID-19, as well as patients on anticoagulation therapy who require regular monitoring of their blood-clotting rates.
Shortages also force providers to evaluate how they buy and use those supplies, which can lead to improvements in test utilization, Pacetti says. For example, “We are doing all of these tests, and 97% of them are normal. Maybe you don’t need to test the PT/INR and PTT on every patient in the ED.”
Gloves are another example. They have been hard to source throughout the pandemic, and those in extra-small and extra-large sizes continue to be in short supply, Pacetti said. As a result of the shortages, staff members, who often have brand preferences, have learned to make do with whatever supplies the hospital finds.
Permanent changes
In addition to changes in test utilization and supply-sourcing strategies, labs have implemented other changes in standard processes.
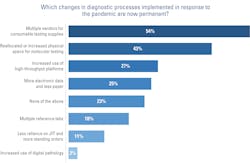
According to respondents to MLO’s SOI survey, some of those changes are likely to become permanent, such as using multiple vendors for consumable testing supplies (54%), reallocating or increasing the physical footprint for molecular testing (43%), increasing the use of high-throughput platforms (27%), and using more electronic data and less paper (25%).
Of those strategies, tapping into multiple vendors for consumables was a strategy that many sizes and types of labs deployed and plan to make permanent, MLO’s SOI survey found.
In terms of hospital labs specifically, half of those surveyed plan to keep the reallocated and/or increased space they have assigned to molecular, while slightly less than one third of them plan to maintain their increased use of high-throughput analyzers, and about one fifth plan to continue relying on multiple reference labs for send-out tests.
Other changes sparked by the pandemic may lead to additional changes in the future. For example, Monument Health is mulling over the idea of developing its own tests for infectious diseases. There are two main advantages to lab-developed tests (LDTs), compared with proprietary tests developed by vendors: more sources for consumable supplies and flexibility to develop tests tailored to the specific needs of the lab and its customers. “I think we would consider that in the future,” DeMenge said.
Clinical Lab Consulting plans to focus on other areas of molecular testing. Says Ingle, “We are deeply entrenched in molecular pathogens, such as UTI and SDI.” The company also is involved in wound care and pharmacogenomics. “COVID was a diversion for us,” he says.
References
- FDA in brief: FDA advises against use of SARS-CoV-2 antibody test results to evaluate immunity or protection from COVID-19, including after vaccination. Food and Drug Administration. May 19-2021. https://www.fda.gov/news-events/press-announcements/fda-brief-fda-advises-against-use-sars-cov-2-antibody-test-results-evaluate-immunity-or-protection. Accessed June 4, 2021.