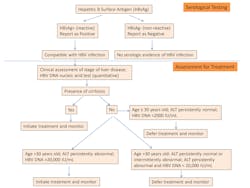
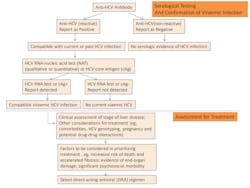
Update on WHO Guidelines for HBV and HCV Testing
Hepatitis B virus (HBV) and Hepatitis C virus (HCV) infections are major causes of acute and chronic liver disease such as cirrhosis and hepatocellular carcinoma (HCC). HCC is the most frequently encountered malignancy throughout the world, resulting in an estimated 1.4 million deaths annually;1 and the incidence of HCC has continued to increase rapidly in the past few decades. HBV is the main cause in endemic regions such as sub-Saharan Africa and Southeast Asia. HCV and steatohepatitis are the main causes in United States, Canada, United Kingdom and Australia.2,3 According to the World Health Organization (WHO), there are 248 million people living with chronic HBV infection; 110 million people are HCV-antibody positive and 80 million people have chronic viraemic HCV infection.1 Even in low-prevalence areas, high levels of HBV and HCV infection are also detected in subpopulations, such as men who have sex with men, individuals who inject drugs, people with HIV as well as indigenous migrants. Given the heavy burden from infections, early detection and prompt treatment are crucial for preventing or delaying the onset of liver disease. The discovery of highly effective oral direct-acting antiviral treatment regimens has revolutionized chronic HCV treatment, with high rates of cure.4
However, despite the heavy burden of disease and the significant advantage of early treatment, most infected people remain unaware of their infection. For example, while the Centers for Disease Control and Prevention (CDC) in August 2012 recommended one-time screening for baby boomers (those born between 1945 and 1965) in United States, until 2013, less than 5 percent of them reported having received the screening. Screening rates remain low and HCV infected individuals are highly undiagnosed.4 The exact hidden burdens from HBV and HCV are very poorly documented, especially in low-income settings. Therefore, accurate testing and diagnosis of HBV and HCV infections has become a critical first step to effective access to both prevention and treatment. It also provides an opportunity to link people to interventions that reduce transmission, through counseling on risk-reduced behaviors and hepatitis B vaccination.
In general, the primary goals of testing from the WHO (modified from the WHO) are as follows:
- To identify infected individuals and their families and treat patients promptly to reduce hepatitis-related mortality. This can be achieved through the use of direct-acting curative antiviral therapy for chronic hepatitis C and lifelong antiviral therapy for chronic hepatitis B infection.
- To monitor the response to antiviral treatment and effectively follow up with these patients and their families.
- To provide preventive interventions to reduce transmission in the community; implement hepatitis B vaccinations and educate the public about hepatitis.
The new guideline: What is the difference?
Due to this global public health problem of HBV and HCV infections, the WHO has developed and implemented both a comprehensive strategy to address this, as well as to provide clear guidance on diagnosis and management. Recent WHO guidelines, however, did not include comprehensive guidance on who to test and how to test for diagnosis. In 2017, the WHO provided the first evidence-based guidance on testing for hepatitis B and C virus infections in adults, adolescents and children, particularly those living in low- and middle-income countries, where the burden of disease is highest and where the treatment is becoming more readily available and affordable. The new guidelines are expected to provide the basis and rationale for the development of national guidelines for hepatitis testing, particularly in resource-limited settings and high-risk subpopulations, with an overall goal to reduce the global burden of HBV and HCV infections.The specific objectives of the guidelines are as follows (modified from the WHO):
- To provide recommendations about who to screen for hepatitis B and hepatitis C infection, and which testing strategies and methods to use.
- To provide summaries of evidence, evaluation of the overall benefits and possible harms, feasibility, costs, and acceptability of the proposed recommendations.
- To provide guidance to support the recommendations at the country level, for example; a systematic approach to optimal assays and evaluation of assays, quality systems for all aspects of hepatitis testing; and a framework for planning the best combination of testing approaches.
- To identify areas for research.
The guiding principles are promoting human rights and equity in access to hepatitis testing; using the public health approach along the continuum of care; and providing accurate testing. All of these should be done according to the “5 Cs”: Consent, Confidentiality, Counseling, Correct test results and Connection (linkage to prevention, treatment and care services).5
How the new changes are related to the lab community
In supporting the implementation of the recommendations from WHO to test for viral hepatitis in different populations, WHO also provides a strategic framework to guide countries’ decision-making on selecting test approaches in different lab settings. And the two key points are as follows (modified from the WHO):
- Viral hepatitis testing can be largely available in different populations and different settings to include both (a) healthcare facility-based testing, which can be found in primary care clincs and outpatient wards and (b) community-based testing, which can be offered through mobile outreach, home-based or door-to-door approaches, in schools, workplaces, parks and other venues.
Countries need to identify the best strategic combination of facility- and community-based testing to best reach out to those with undiagnosed infections as well as populations at high risk.
To assess and improve the selection of hepatitis testing approaches, the first step is to review national and sub-national epidemiology goals and set testing (and treatment) coverage targets. The next step is to review the effectiveness of existing testing services and identify the gaps and assess the costs and cost-effectiveness of different testing approaches. The final mix of testing approaches, with the greatest public health benefit and impact, should be based on a situational assessment. Overall, a mix of hepatitis testing approaches that are focused on populations and/or geographic locations with high HBV or HCV prevalence are likely to have the greatest impact, be the most cost-effective and have the most beneficial outcomes. At the same time, it is also very important to monitor, evaluate and adjust testing program activities to accommodate specific needs.
References
- Guidelines for the screening, care and treatment of persons with chronic hepatitis C infection. Updated version, April 2016. Geneva: World Health Organization; 2016.
- Davila JA., et al., Hepatitis C infection and the increasing incidence of hepatocellular carcinoma: a population-based study. Gastroenterology. 2004;127(5): p. 1372-80
- Siegel RL., Miller KD, A. Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018; 68(1): p. 7-30.
- Manjelievskaia J, Brown D, Shriver CD, Zhu K.CDC Screening Recommendation for Baby Boomers and Hepatitis C Virus Testing in the US Military Health System. Public Health Rep. 2017 Sep/Oct;132(5):579-584.
- Consolidated guidelines on HIV testing services. Geneva: World Health Organization; 2015 http://apps. who.int/iris/bitstream/10665/179870/1/9789241508926_ eng.pdf?ua=1&ua=1, 6 Accessed February 2017.
About the Author

(SB) Shi Bai, MD, PhD
is the chief resident of the Department of Pathology at University of Massachusetts (UMass) Memorial Medical Center. Her research focus is diagnosis of cancer and non-cancer diseases based on microscopic and biomedical examinations.

(SF) Shu-Ling Fan, PhD
is the director of clinical laboratories and point-of-care testing at UMass Memorial Medical Center, Worcester, MA. Her research focus is biomarker development for disease diagnosis. She is fully committed to providing quality results to support clinical diagnosis.