The goal of any clinical diagnostic test procedure is to provide critical information in a timely manner so that appropriate actions may be taken, ultimately improving patient outcomes. Point-of-care testing (POCT) is a term that has come to describe a multitude of rapid medical tests that can be performed at or near the site of patient care. The most compelling benefit of these tests is that, as opposed to having to wait hours or days for results to arrive from an outside laboratory, clinicians can obtain the results immediately, allowing for clinical management decisions to be made while the patient is still at the care facility. While the implementation of rapid diagnostic tests dates back to ancient history (sweet-tasting urine was once commonly used to diagnose diabetes mellitus), it was not until the 1950s that these rapid diagnostic methods gained any real predictive value. Today, the popularity and demand for POCT are increasing rapidly. TriMark Publications estimates that the global market for POCT was $14.5 billion in 2016, and is expected to grow by seven percent over the next five years.1
Urinalysis dipsticks at the point-of-care
Urinalysis using multi-analyte dipsticks is a point-of-care test performed at any hospital, clinical laboratory, doctor’s office, health clinic, and nursing facility. Various iterations of these tests have existed for decades, and they continue to be among the most commonly performed tests of any kind. Urinalysis dipsticks contain discrete reagent pads to semi-quantitatively test for the presence of bilirubin, blood, creatinine, glucose, ketones, leukocytes, nitrite, pH, protein, specific gravity, and urobilinogen in a urine sample. Some urinalysis dipsticks contain reagent pads to test for the presence of creatinine and microalbumin. These tests may be read visually by comparing the colors that develop on each reagent pad to a chart provided by the strip manufacturer, or by an automated urine dipstick analyzer which helps to provide consistency in the timing and color interpretation regardless of lighting conditions or personnel.
Overview of QC for urinalysis dipsticks
Running daily Quality Control (QC) for POCT is critical. When measuring any kind of patient sample for indicators of disease, stable controls must be used to validate instrument performance and ensure accurate patient diagnosis. Using QC materials is not only good practice for labs that test human samples, but is also the law per the regulations outlined by the Clinical Laboratory Improvement Amendments (CLIA). Per CLIA 42 CFR section 493.1256 – Standard: Control Procedures: a) For each test system, the laboratory is responsible for having control procedures that monitor the accuracy and precision of the complete analytical process.2
Every facility in the United States that performs testing on human specimens for health assessment or the diagnosis, prevention, or treatment of disease is regulated under CLIA. Clinical tests are categorized as either waived, moderate, or high complexity. What category a test falls under depends on the amount of training required to perform the test, the degree of interpretation and judgment required, the difficulty of calculations, calibration and quality control requirements. Generally, CLIA-waived tests are considered the least likely to give an erroneous result. In the event of an erroneous result, they are the least likely to pose serious harm to the patient.
There is no guarantee that CLIA-waived tests will be completely error-free, however, and a bad result can lead to a misdiagnosis and mistreatment. In fact, a study conducted across three hospitals in the United Kingdom in 2009 and 2010 determined that POCT represented error rates that were considerably higher than central laboratory testing and that most of the errors occurred in the analytical phase.3 The College of America Pathologists Laboratory Accreditation Program (CAP-LAP) states that all clinical laboratory tests, including CLIA-waived tests, should follow a routine QC program as per other moderate and high complexity tests.4
Urinalysis dipsticks fall into the CLIA-waived category and are generally very reliable, simple to use, and easy to interpret. There are, however, numerous potential scenarios where a competent user may obtain an erroneous result. For example, most manufacturers package urinalysis dipsticks in canisters with a desiccant to keep the reagent pads dry. Failure to close the canister correctly can result in ambient moisture affecting the performance of the test. The leukocyte reagent pad, for instance, is particularly sensitive to humidity, and a poorly stored dipstick can lead to a false-negative leukocyte result, thereby missing a diagnosis for a potential urinary tract infection. Prolonged exposure to high temperatures and light can also negatively affect the performance of the tests.
Dipper or dropper?
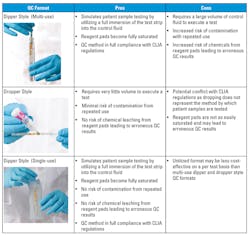
When it comes to selecting QC for urinalysis dipstick testing, the two main formats to consider are dipper- and dropper-style controls. As the names imply, a dipper style control is used by fully immersing the urinalysis dipstick into the control fluid to fully saturate the reagent pads, whereas a dropper-style control is used by dispensing the control fluid dropwise onto the reagent pads. Several manufacturers produce urinalysis dipstick controls in these two basic formats, each with a unique set of stability claims, features, and advantages. Dipper-style controls are typically delivered in tubes with 10-15 mL of fluid. The minimum amount of fluid required to execute a test in a standard 13 x 100 mm borosilicate test tube is about 8.5 mL. This is quite a large volume of control per test, but it is necessary in this format to ensure that reagent pads are immersed.
Single-use dipper-style controls in this format would therefore be rather cost-prohibitive, which is why many control manufacturers allow for multiple dips into the same control tube. There is, however, a limit to the number of tests that can be performed in the same tube, because a variety of chemicals leach out of the reagent pads, potentially leading to erroneous QC results. The blood analyte reaction is particularly sensitive to shifts in pH and exposure to oxidative compounds that become released from the reagent pads. This effect is exacerbated by repeated dips over extended periods of time. Repeated use of this style of control also increases the risk of microbial contamination from frequent handling and multiple testing events.
Dropper-style controls are the most cost-effective because very little volume is required to execute a test. As many of the new generation of urinalysis dipsticks are formulated with specialized reagent pads that help prevent carryover contamination to neighboring reagent pads, they may sometimes be more difficult to fully saturate using a dropper-style control. The drops of control fluid tend to sit on top of the reagent pad until enough material has been delivered to fully penetrate. Failure to thoroughly wet the reagent pad with the control fluid may lead to an erroneous QC result. The glucose reagent pad on some brands of urinalysis dipsticks is particularly difficult to saturate using a dropper-style control because manufacturers have taken steps to prevent the reagents from carrying over to other pads. More specifically, the peroxidase enzyme from the glucose reagant pad can trigger a false-positive result on the blood reagent pad.
This issue can be mitigated by implementing proper training when utilizing dropper-style QC for urinalysis. There may be some confusion and/or lack of consensus as to how to interpret CLIA regulations when using dropper-style QC. CLIA 42 CFR section 493.1256 states, “(8) Test control materials in the same manner as patient specimens.”2 Urinalysis dipsticks are intended to be dipped into the patient’s urine sample, fully immersing the reagent pads. Nonetheless, there are some legitimate scenarios, such as in cases of low sample volume or with neonatal urine samples, that a dropping method may be utilized with patient samples.
Single-use dipper QC
Given the pros and cons of the dipper- and dropper-style controls (Table 1), the ideal control would be comprised of the best aspects of each: full immersion for pad saturation and CLIA compliance, and reduced risk for reagent pad leaching and contamination from repeated use. Since refrigeration is not always available near the site of patient care, many POCT devices are designed to be stored and operated at room temperature (RT).
Consequently, QC materials that are used to verify the performance of the POCT devices would ideally also have extended RT stability. It follows that a single-use dipper style control, with extended RT stability, would be the ideal solution for urinalysis dipsticks QC performed at the point-of-care, particularly if the large volume requirement can be significantly reduced. A U.S. patent5 has recently been granted for such a device whereby the control fluid is contained within a thermoplastic pouch that allows for the full immersion of a urinalysis dipstick in only 1.5 mL of control fluid, a mere fraction of the volume required for the traditional dipper style. Moreover, the single-use nature of the new format mitigates the risks associated with repeated use. Most important, this format directly simulates the analytical process used to test patient samples, thus providing the most robust and appropriate form of QC for urinalysis dipsticks for any clinical setting.
REFERENCES
- Point of Care Diagnostic Testing World Markets, TriMark Publications, May 2017.
- Clinical Laboratory Improvement Amendments, §493.1256 Standard: Control
Procedures. - O’Kane MJ, McManus P, McGowan N, Lynch PL. Quality error rates in point-of-care testing. Clin Chem. 2011;57(9):1267-1271.
- Laboratory Accreditation Program. College of American Pathologists (CAP).
Northfield, IL, 1997. - Ban MS, Fernández BR, et.al. US Patent #14/745,675. Liquid Holding Apparatus for Insertion of a Test Device into a Test Liquid. 2017.
Brian Fernández serves as Director of Research and Development for Redondo Beach, California-based Quantimetrix.
About the Author

Brian Fernandez
serves as Director of Research and Development for Redondo Beach, California-based Quantimetrix.