Optimizing C. difficile testing in a large system lab by integrating inpatient and outpatient needs
HealthPartners is an integrated healthcare organization with more than 1,700 physicians caring for patients in 100 medical and dental clinics, including more than 50 primary care clinics, six hospitals, and numerous specialty centers in the greater Minneapolis-St. Paul metropolitan area and western Wisconsin. More than 35 laboratories, located in clinics and hospitals, offer specimen collection and other services, while the Regions Hospital laboratory in St. Paul is the centralized testing location for outpatient Clostridium difficile testing.
In 2016, the Regions Hospital laboratory performed close to 7,500 tests for C. difficile, of which 26 percent were for inpatients while the remaining were from outpatient clinics. To address the varying inpatient and outpatient needs of the health system, all with the common goals of timely treatment and prevention, as well as cost efficiency, HealthPartners developed an integrated inpatient/outpatient strategy for C. difficile testing. The planning and implementation process yielded several valuable insights for our C. difficile testing options.
Overview of CDI
Clostridium difficile is a spore-forming, Gram-positive anaerobic bacteria and a significant cause of healthcare-associated infections (HAIs).1 C. difficile infection (CDI) symptoms include watery diarrhea and mild abdominal cramping and tenderness. Severe infection results in watery diarrhea multiple times per day, severe abdominal cramping, colitis, pseudomembranous colitis, and in rare cases, toxic megacolon. About half a million people in the United States are infected each year, with a mortality rate of five percent and estimated healthcare costs of $4.8 billion just for acute care facilities.2
Antibiotics are a major risk for the development of CDI because the resulting loss of endogenous microbiota allows the pathogen, transmitted via the fecal-oral route, to proliferate. Patients most at risk are the elderly, especially those using antibiotics. Increasingly, CDI is reported in the community setting.3 Also noteworthy is the emergence of the current epidemic strain: BI/NAP1/027. Infections with this C. difficile strain are characterized by increased production of both toxins A and B and an additional toxin known as binary toxin. This hypervirulent strain causes efficient sporulation and high-level resistance to fluoroquinolones, the antibiotics commonly used in treating CDI, and higher mortality rates.4
Diagnosing CDI
Timely diagnosis and isolation of infected hospitalized patients is critical to management of CDI. “Sniff” testing based on the smell of stool has been shown to be an inaccurate diagnostic tool.5 Enzyme immunoassay (EIA) tests for toxins in stool were once widely used, but due to lack of sensitivity are no longer supported by guidelines as a standalone assay.6 Glutamate dehydrogenase, an enzyme produced by C. difficile in relatively large amounts, has been used as a screening test, but this test lacks specificity. Cell culture cytotoxicity neutralization assay (CCCNA) was the gold standard, but is not suitable for routine use because of the relatively prolonged turnaround time (24 to 48 hours), and the requirement for expertise in maintenance of cell cultures and interpretation of results. Stool culture is not widely available because of both the sensitivity issues and complexity requirements for anaerobic culture.
The nucleic acid amplification test (NAAT) is the standard of care today, with superior sensitivity and specificity and quick turnaround.4,6,7 In particular, use of a polymerase chain reaction (PCR) test to rule out C. difficile infection has been shown to reduce duration of empiric antibiotic therapy and improve timely isolation of inpatients.8 Current NAATs target the tcdB gene, which is produced by all human disease-causing strains of C. difficile, and is the most reliable region for identifying C. difficile as the causal agent for symptomatic patients.9,10
Colonization vs. infection
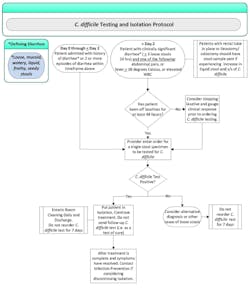
One important consideration in C. difficile testing is false positives, since laboratory testing cannot distinguish between asymptomatic colonization and symptomatic infection with C. difficile. To avoid false-positive results and unnecessary isolation and treatment, CDI testing is performed only on patients with clinically significant diarrhea (three or more loose stools within 24 hours). Inpatient testing is further restricted in that patients admitted without diarrhea must have documentation of no prior use of laxatives and be >2 days post-admission prior to testing. In general, testing in children under three years of age should be avoided due to prevalence of C. difficile colonization.11 (Visit www.mlo-online.com to view a flow chart illustrating the protocol at Regions Hospital for identifying inpatients for testing.) A rapid PCR test with a turnaround time (TAT) of <1 hour is offered to inpatients to expedite testing. Outpatient C. difficile testing is batch-tested with a same-day turnaround time (extended on weekends).Strategies for success
The HealthPartners system includes both inpatient and outpatient testing, and we always look for opportunities to optimize our testing resources. Our hospitals use PCR for C. difficile testing, and a one-hour turnaround time is paramount both for care of CDI patients and infection control. However, inpatient testing is only a part of the overall CDI testing need. About 5,000 C. difficile test orders per year also arrive from our outpatient clinics. As a system, we reviewed our inpatient and outpatient testing needs with three goals in mind: 1) deliver quality care; 2) create a positive experience for the patient and healthcare team; and 3) maintain cost-effective testing.
With regard to addressing inpatient vs. outpatient needs, we identified three requirements for inpatient CDI testing: 1) assay performance: a sensitive, specific assay with a reliable negative predictive value; 2) rapid turnaround time (<1 hour), necessary to promote patient care and reduce transmission; 3) complexity: optimize staffing and training with an easy-to-use assay.
Outpatient testing shares the need for performance (sensitivity, specificity) and an easy-to-use system. However, the turnaround time expectation for outpatient is less stringent; same-day results are adequate for patient care needs. The outpatient testing algorithm provides us the opportunity to improve cost-effectiveness by using a fully automated batch testing system. Ordering tests and reporting results are expeditious with our information system capabilities.
Leveraging the capacity of systems is also a key part of our success. When the lab team began to formulate C. difficile testing strategies, standardization and centralization for outpatient testing made sense for our system. Key considerations were leveraging testing systems already in place that had the capacity for menu extension, and tailoring testing to support patient care. At the time, the Regions Hospital laboratory was using an automated PCR system for human papillomavirus (HPV) testing.
C. difficile testing was also available on the existing platform, so it made sense for our outpatient testing needs to add the test to the already existing platform. No additional capital acquisition was required and operator training was minimal, allowing for a cost-effective method to consolidate outpatient C. difficile testing. The turnaround time of less than 24 hours, except on weekends, fits the outpatient clinical workflow and meets physicians’ and patients’ expectations.
As is customary within our system, initial planning and testing strategy involved laboratory, medical-technical and operations teams, infectious disease physicians, gastroenterology physicians, infection prevention, and executive management. In developing the strategy, the team considered the implications for both clinical and laboratory process and workflow. Inpatient C. difficile testing remained on site to ensure a one-hour turnaround time. Outpatient testing was consolidated to the Regions Hospital laboratory, which tripled the testing volume for that location. The laboratory team optimized specimen workflow and absorbed the 5,000-plus outpatient C. difficile tests without adding additional instrumentation or staff.
Our laboratory teams now work closely to review workflows and identify gaps in specimen transport, testing, and result reporting, particularly in locations that serve as centralized testing laboratories. In addition, our inpatient infection prevention teams re-evaluate C. difficile order protocols to ensure that we’re testing appropriately.
Summary
The two-pronged testing strategy (inpatient 1 hour TAT, outpatient same-day TAT) we applied for C. difficile testing helped us meet our three goals. Our strategy addresses the diverse needs of inpatients with a higher likelihood of CDI and thus urgent need for treatment and isolation, and outpatient testing.
REFERENCES
- Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of health care–associated infections. N Engl J Med. 2014;370(13):1198-1208.
- Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372(9):825-834.
- Jury LA, Sitzlar B, Kundrapu S, et al. Outpatient healthcare settings and transmission of Clostridium difficile. PLoS One. 2013;8(7):e70175. Epub 2013 Jul 24.
- Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med. 2015;372(16):1539-1548.
- Rao K, Berland D, Young C, Walk ST, Newton DW. The nose knows not: poor predictive value of stool sample odor for detection of Clostridium difficile. Clin Infect Dis 2013;56(4):615–616.
- Dubberke ER, Carling P, Carrico R, et al. Strategies to prevent Clostridium difficile infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(6):628-645.
- Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108(4):478-498.
- Peppard WJ, Ledeboer NA. Implementation of polymerase chain reaction to rule out Clostridium difficile infection is associated with reduced empiric antibiotic duration of therapy. Hosp Pharm.2014;49(7):639-643.
- Drudy D, Fanning S, Kyne L. Toxin A-negative, toxin B-positive Clostridium difficile. Int J Infect Dis. 2007;11(1):5-10.
- Lyras D, O’Connor JR, Howarth PM, et al. Toxin B is essential for virulence of Clostridium difficile. Nature. 2009;458(7242):1176-1179. doi: 10.1038/nature07822. Epub 2009 Mar 1.
- Churgay CA, Aftab Z. Gastroenteritis in children: Part 1. Diagnosis. Am Fam Physician. 2012;85(11):1059-1062.
Nicole Zitterkopf Khoury, PhD, D(ABMM), MPH, is Laboratory Scientific and
Accreditation Director at HealthPartners and Park Nicollet Medical Groups
(Bloomington, MN).